A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
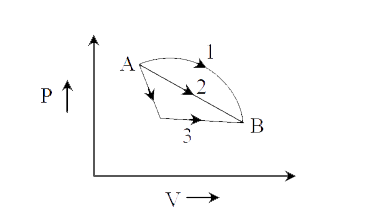
- An ideal gas goes from state A to state B via three different processe...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- A gas is expanded form volume V(0) to 2V(0) under three different proc...
Text Solution
|
- दिखाए गए P-V आरेख के अनुसार एक आदर्श गैस को तीन विभिन्न प्रक्रमों द्...
Text Solution
|
- The initial state of 1 mol of an ideal gas is (P(1),V(1),T(1)) . The g...
Text Solution
|
- m द्रव्यमान की एक आदर्श गैस चित्र में प्रदर्शित स्थिति A से B तक तीन व...
Text Solution
|
- A gas is expanded from volume V(0) to 2V(0) under three different proc...
Text Solution
|
- An ideal gas goes from state A to state B via three different processe...
Text Solution
|