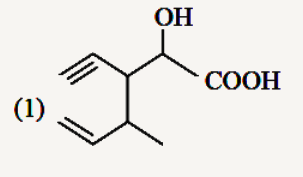
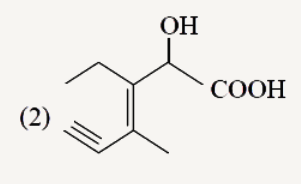
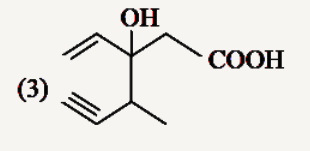
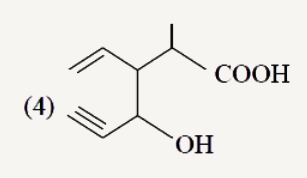
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-meth...
Text Solution
|
- Structure of the compound whose IUPAC name is 3- ethyl -2- hydroxy -4-...
Text Solution
|
- Structure of the compund whose IUPAC name is 3 -ethyl- 2 -hydroxy- 4 -...
Text Solution
|
- 3- Ethyl -2- Hydroxy -4- methylhex -3- en -3- en -5- ynoic acid :-
Text Solution
|
- Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-meth...
Text Solution
|
- Structure of the compund whose IUPAC name is 3-ethyl-2-hydroxy-4-methy...
Text Solution
|
- Structure of the compound whose IUPAC name is 3-ethyl. 2-hydroxy-4-met...
Text Solution
|
- Stucture of the compound whose IUPAC name is 3 - ethyl - 2 - hydroxy -...
Text Solution
|
- Which one of the following structures has the IUPAC name 3 - ethynyl -...
Text Solution
|