A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Number of possible isomers for the complex [Co(en)(2)Cl(2)]Cl will be ...
Text Solution
|
- Number of possible isomer for the complex [Co(en)(2)CI(2)]CI will be: ...
Text Solution
|
- How many isomers are possible for the complex [Co(en)(2)Cl(2)](en=ethy...
Text Solution
|
- Number of possible isomers for the complex [Co(en)(2)CI(2)] will be (...
Text Solution
|
- Number of possible isomer for the complex [Co(en)(2)CI(2)]CI will be: ...
Text Solution
|
- Number of possible isomers for the complex [Co(en)(2)Cl(2)] will be (e...
Text Solution
|
- Number of possible isomers for the complex [Co(en)(2)CI(2)] will be (e...
Text Solution
|
- संकुल [Co("en")(2)Cl(2)]Cl के संभावित समवयवो की संख्या होगी (en= एथिली...
Text Solution
|
- Number of possible isomers for the complex [Co(en)(2)Cl(2)]Cl will be
Text Solution
|
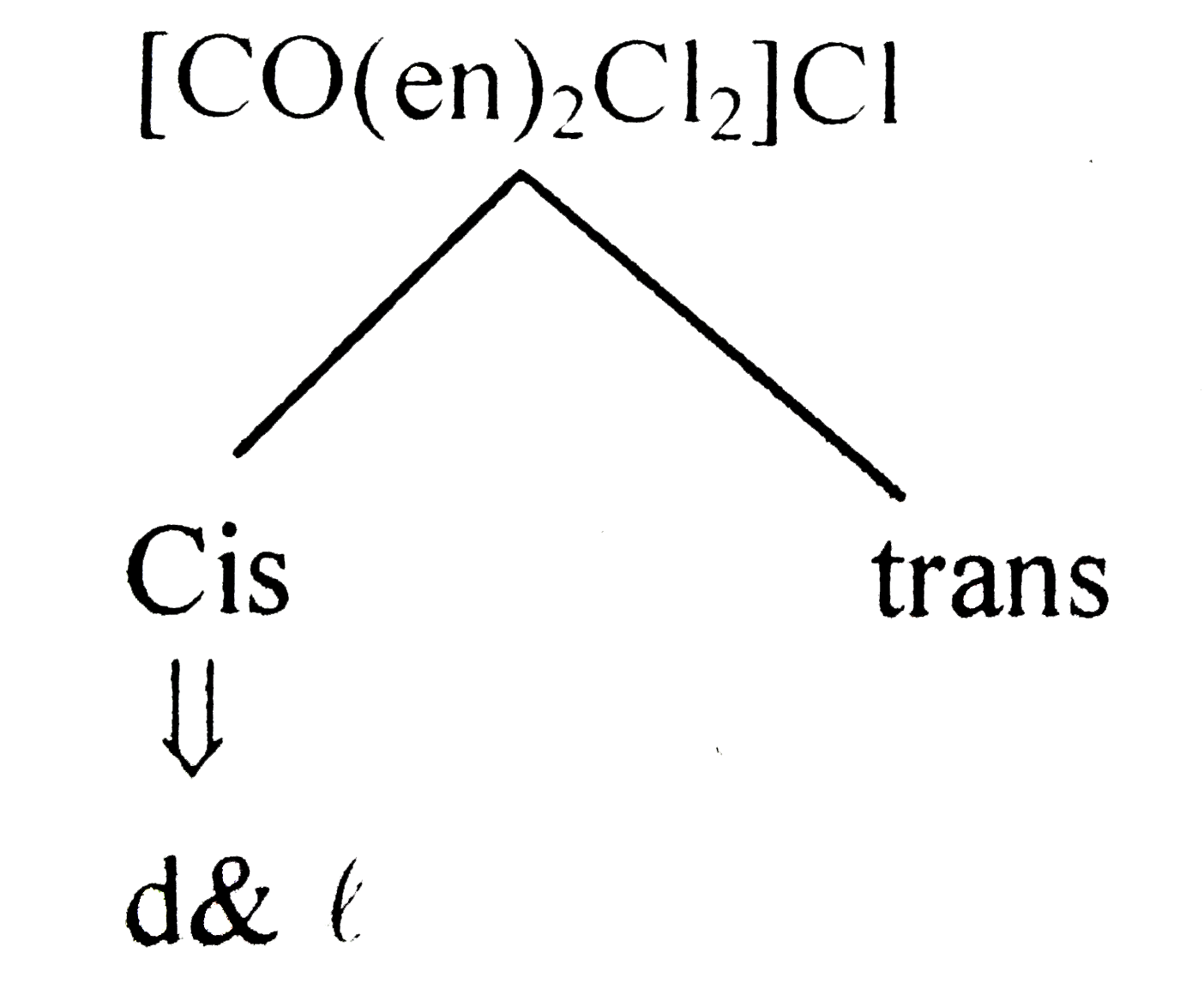
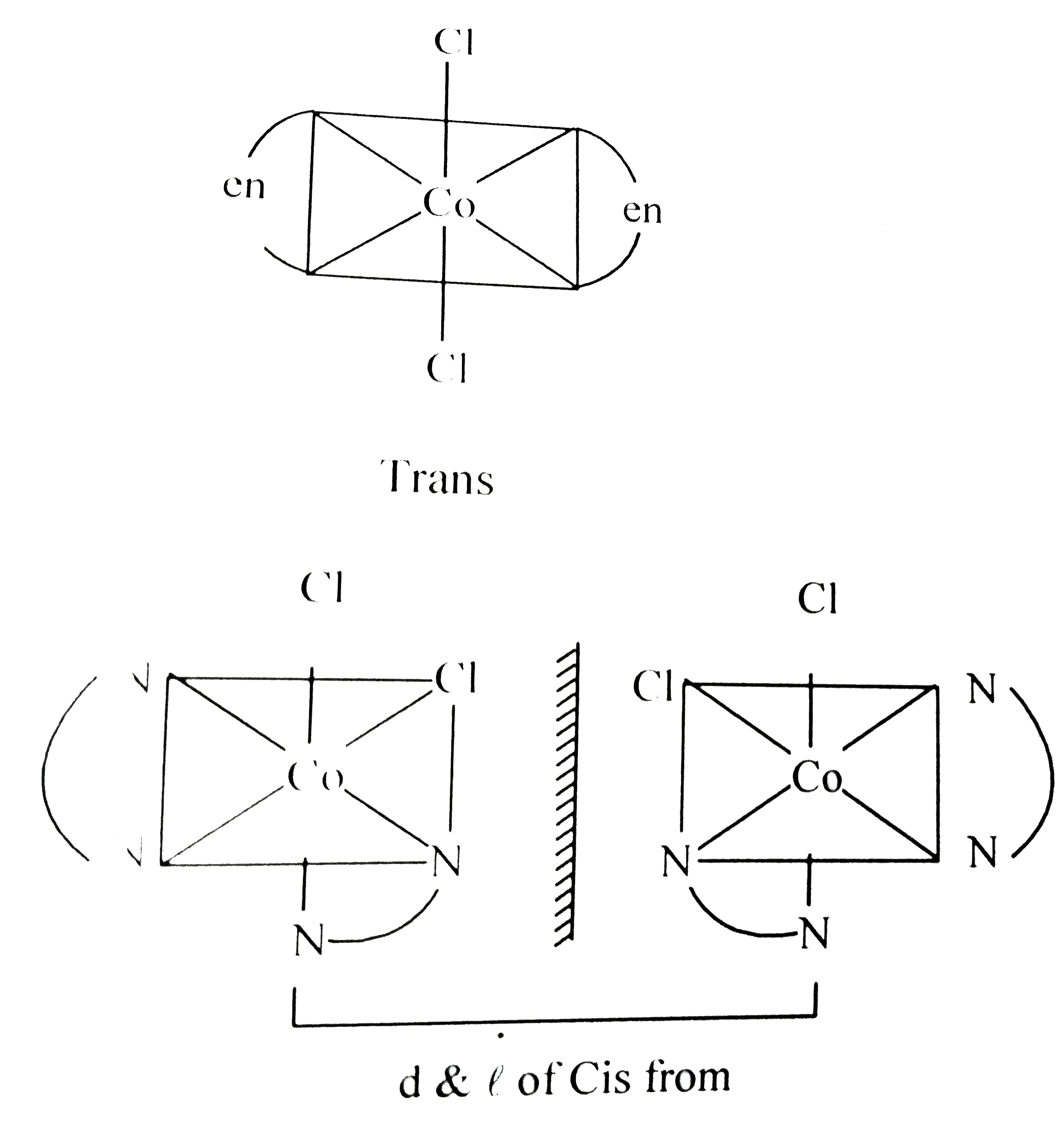 .
.