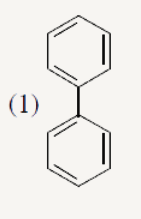
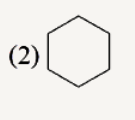
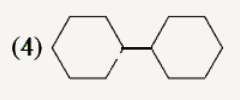
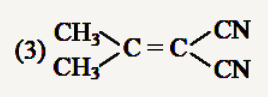A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following moleucles, all atoms are coplanar?
Text Solution
|
- Which moleucle is T-shaped ?
Text Solution
|
- In which of the following moleucles are all the bonds not equal ?
Text Solution
|
- In which of the following moleucles, all atoms are coplanar?
Text Solution
|
- The molecule in which all atoms are not coplanar is
Text Solution
|
- In which of the following molecules, all atoms are coplanar ?
Text Solution
|
- Assertion : All collision of reactant molecule lead to product formati...
Text Solution
|
- In which of the following molecules, all the atoms are coplanar-
Text Solution
|
- In which of the following molecules, all atoms are coplanar ?
Text Solution
|