Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-CHEMISTRY 2011-SECTION-II
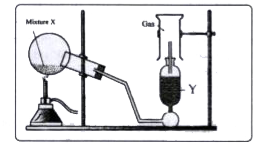
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- The diagram shows an experimental set up for the laboratory preparatio...
Text Solution
|
- An organic compound with vapour density = 94 contains C=12.67%, H = ...
Text Solution
|
- Calcuate the mass of 10^(22) atoms of sulphur. [ Atocmi mass S=32,...
Text Solution
|
- Calculate the mass of 0.1 mole of carbon dioxide. [Atomic mass : S...
Text Solution
|
- In the laboratory preparation of hydrochloric acid, HCl gas is dissolv...
Text Solution
|
- In the laboratory preparation of hydrochloric acid, HCl gas is dissolv...
Text Solution
|
- In the laboratory preparation of hydrochloric acid, HCl gas is dissolv...
Text Solution
|
- In the laboratory preparation of hydrochloric acid, HCl gas is dissolv...
Text Solution
|
- Choose the correct word/phrase from within the brackets to complete th...
Text Solution
|
- When acetaldehyde is oxidised with acidified potassium dichromate, it ...
Text Solution
|
- Choose the correct word/phrase from within the brackets to complete th...
Text Solution
|
- Choose the correct word/phrase from within the brackets to complete th...
Text Solution
|
- Choose the correct word/phrase from within the brackets to complete th...
Text Solution
|
- Write balanced chemical equations for the following: Monochloro etha...
Text Solution
|
- Write balanced chemical equations for the following: A mixture of so...
Text Solution
|
- Write balanced chemical equations for the Ethanol under high pressur...
Text Solution
|