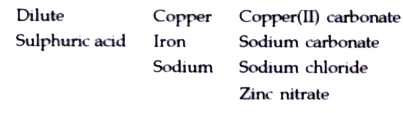Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-CHEMISTRY-2013-SECTION-II (40 Marks)
- Compare the compounds methane and sodium chloride with regard to solub...
Text Solution
|
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Choosing the substances from the list given below, write balanced chem...
Text Solution
|
- Write balanced chemical equations for the reactions which would be use...
Text Solution
|
- State two relevant observation Ammonium hydroxide solution is added ...
Text Solution
|
- State two relevant observation Ammonium hydroxide solution is added...
Text Solution
|
- State two relevant observation Lead nitrate crystals are heated in a...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Using the information above, complete the following: .......... is ...
Text Solution
|
- Using the information above, complete the following: Metal atoms te...
Text Solution
|
- Using the information above, complete the following: Non-metallic ...
Text Solution
|
- Using the information above, complete the following: Non-metallic e...
Text Solution
|
- Using the information above, complete the following: Metals tend to...
Text Solution
|
- Give balanced equations for Reduction of hot copper (II) oxide to c...
Text Solution
|
- Give balanced equations for Oxidation of carbon with concentrated ni...
Text Solution
|
- Give balanced equations for Dehydration of concentrated sulphuric ac...
Text Solution
|
- Copy and complete the following table relating to important industrial...
Text Solution
|