Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-CHEMISTRY-2013-SECTION-II (40 Marks)
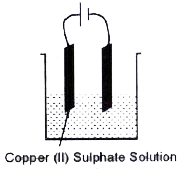
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Copper sulphate solution is electrolysed using copper electrodes. Stud...
Text Solution
|
- Using the information above, complete the following: .......... is ...
Text Solution
|
- Using the information above, complete the following: Metal atoms te...
Text Solution
|
- Using the information above, complete the following: Non-metallic ...
Text Solution
|
- Using the information above, complete the following: Non-metallic e...
Text Solution
|
- Using the information above, complete the following: Metals tend to...
Text Solution
|
- Give balanced equations for Reduction of hot copper (II) oxide to c...
Text Solution
|
- Give balanced equations for Oxidation of carbon with concentrated ni...
Text Solution
|
- Give balanced equations for Dehydration of concentrated sulphuric ac...
Text Solution
|
- Copy and complete the following table relating to important industrial...
Text Solution
|
- The following questions relate to the extraction of aluminium by elect...
Text Solution
|
- The following questions relate to the extraction of aluminium by elect...
Text Solution
|
- The following questions relate to the extraction of aluminium by elect...
Text Solution
|
- Give balanced chemical equation for the preparation of the following o...
Text Solution
|
- Give balanced equations for the laboratory preparations of butene from...
Text Solution
|
- Give balanced equations for the laboratory preparations of the organi...
Text Solution
|
- Give balanced equations for the laboratory preparations of. i] A satu...
Text Solution
|
- Give the structural formula of each of the isomer of butane
Text Solution
|