Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SELF ASSESSMENT PAPER 4-Section -II
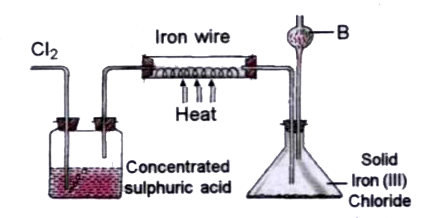
- The diagram given below is to prepare Iron (III) chloride in the labor...
Text Solution
|
- The diagram given below is to prepare Iron (III) chloride in the labor...
Text Solution
|
- The diagram given below is to prepare Iron (III) chloride in the labor...
Text Solution
|
- Study the figure given below and answer the question that follow : ...
Text Solution
|
- Study the figure given below and answer the questions that follow : ...
Text Solution
|
- Study the figure given below and answer the questions that follow : ...
Text Solution
|
- By drawing an electron dot diagram, show the lone pair effect leading...
Text Solution
|
- Write the equations for sulphur combining directly with : A metal .
Text Solution
|
- Write the equations for sulphur combining directly with : A non-meta...
Text Solution
|
- State the conc . Acid which will oxidise sulphur directly to H(2)SO(4)...
Text Solution
|
- What is the name of the process by which sulphuric acid is manufacture...
Text Solution
|
- Concentrated sulphuric acid is used in the laboratory preparation of n...
Text Solution
|
- Determine the empirical formula of a compound containing 47.9% potassi...
Text Solution
|
- Calculate the percentage of Sodium in Sodium aluminium fluoride. (Na3 ...
Text Solution
|
- Match the following:
Text Solution
|
- A metal A in the form of turnings reacted with a hot concentrated diba...
Text Solution
|
- Give reasons why? Aluminium cannot be obtained by the reduction of i...
Text Solution
|
- Name the property for which aluminium is used in cooking utensils.
Text Solution
|
- Why do covalent compounds exist as gases, liquids or soft solids?
Text Solution
|
- Show the bond formation in ammonia and name the bond.
Text Solution
|