Similar Questions
Explore conceptually related problems
Recommended Questions
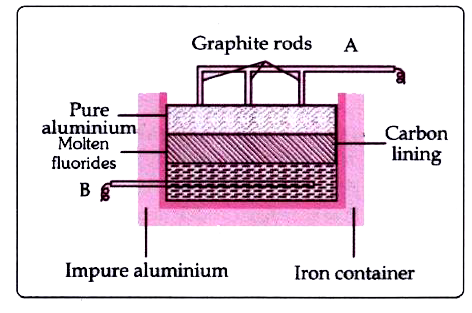
- The sketch below illustrates the refining of aluminium by Hoope's proc...
Text Solution
|
- Metal which cannot be refined by electrolytic refining process is
Text Solution
|
- During Hoope's process for eelctrolytic refining of Al, the middle lay...
Text Solution
|
- Purification of aluminium by electrolytic refining is carried out by :
Text Solution
|
- वैद्युत अपघटनी शोधन प्रक्रम में-
Text Solution
|
- Hoope's process is used in the refining of
Text Solution
|
- Purification of aluminium by electrolytic refining is carried out by
Text Solution
|
- Purification of aluminium done by electrolytic refining is known as
Text Solution
|
- During Hoope's process for electrolytic refining of Al, the middle lay...
Text Solution
|