Topper's Solved these Questions
SAMPLE QUESTION PAPER 1
ICSE|Exercise PART -II (SECTION-B)|7 VideosSAMPLE QUESTION PAPER 1
ICSE|Exercise PART -II (SECTION-C)|9 VideosSAMPLE QUESTION PAPER 1
ICSE|Exercise PART -II (SECTION-C)|9 VideosSAMPLE QUESTION PAPER -2
ICSE|Exercise QUESTION|68 VideosSAMPLE QUESTION PAPER 3
ICSE|Exercise QUESTION|70 Videos
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 1-PART -II (SECTION-A)
- Determine the freezing point of a solution containing 0.625 g of gluco...
Text Solution
|
- A 0.15 M aqueous solution of KCl exerts an osmotic pressure of 6.8 atm...
Text Solution
|
- A solution containing 8.44 g of sucrose in 100 g of water has a vapour...
Text Solution
|
- When ammonium chloride and ammonium hydroxide are added to a solution ...
Text Solution
|
- A solution of potassium chloride has no effect on litmus whereas, a so...
Text Solution
|
- How many sodium ions and chloride ions are present in a unit cell of s...
Text Solution
|
- Lead sulphide has face centred cubic crystal structure. If the edge le...
Text Solution
|
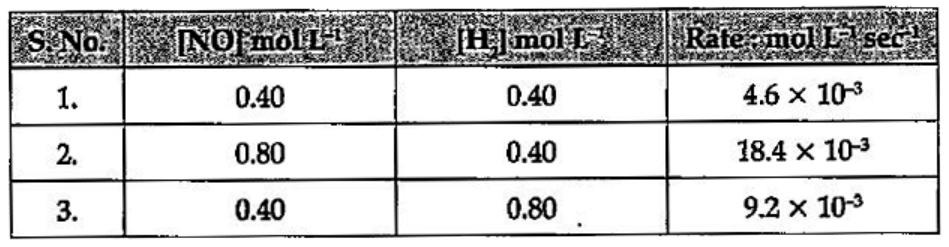
- For the reaction: 2H2 + 2NO <implies 2H(2)O + N(2), the following rat...
Text Solution
|
- The following electrochemical cell is set up at 298 K: Zn//Zn^(2+) (...
Text Solution
|
- (ii) Answer the following: (1) What is the effect of temperature on...
Text Solution
|
- Schottky defect lowers the density of ionic crystals while Frenkel def...
Text Solution
|
- Name the law or principle to which the following observations conform ...
Text Solution
|
- Write two differences between 'order of reaction' and 'molecularity of...
Text Solution
|
- Explain why high pressure is required in" the manufacture of sulphur t...
Text Solution
|
- Calculate the equilibrium constant (K) for the formation of NH^ in the...
Text Solution
|
- Explain the following: Hydrolysis of ester (ethyl acetate) begins slo...
Text Solution
|
- Assertion : The pH of an aqueous solution of acetic acid remains uncha...
Text Solution
|