Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
ICSE|Exercise Follow up Problems|48 VideosELECTROCHEMISTRY
ICSE|Exercise EXERCISE (PART-I Objective Questions)|28 VideosDISTINCTION BETWEEN PAIRS OF COMPOUNDS
ICSE|Exercise QUESTIONS |155 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
ICSE|Exercise EXERCISE (PART-I ( OBJECTIVE QUESTIONS )) (Fill in the blanks)|60 Videos
ICSE-ELECTROCHEMISTRY-ISC EXAMINATION QUESTIONS (PART-II Numerical Problems)
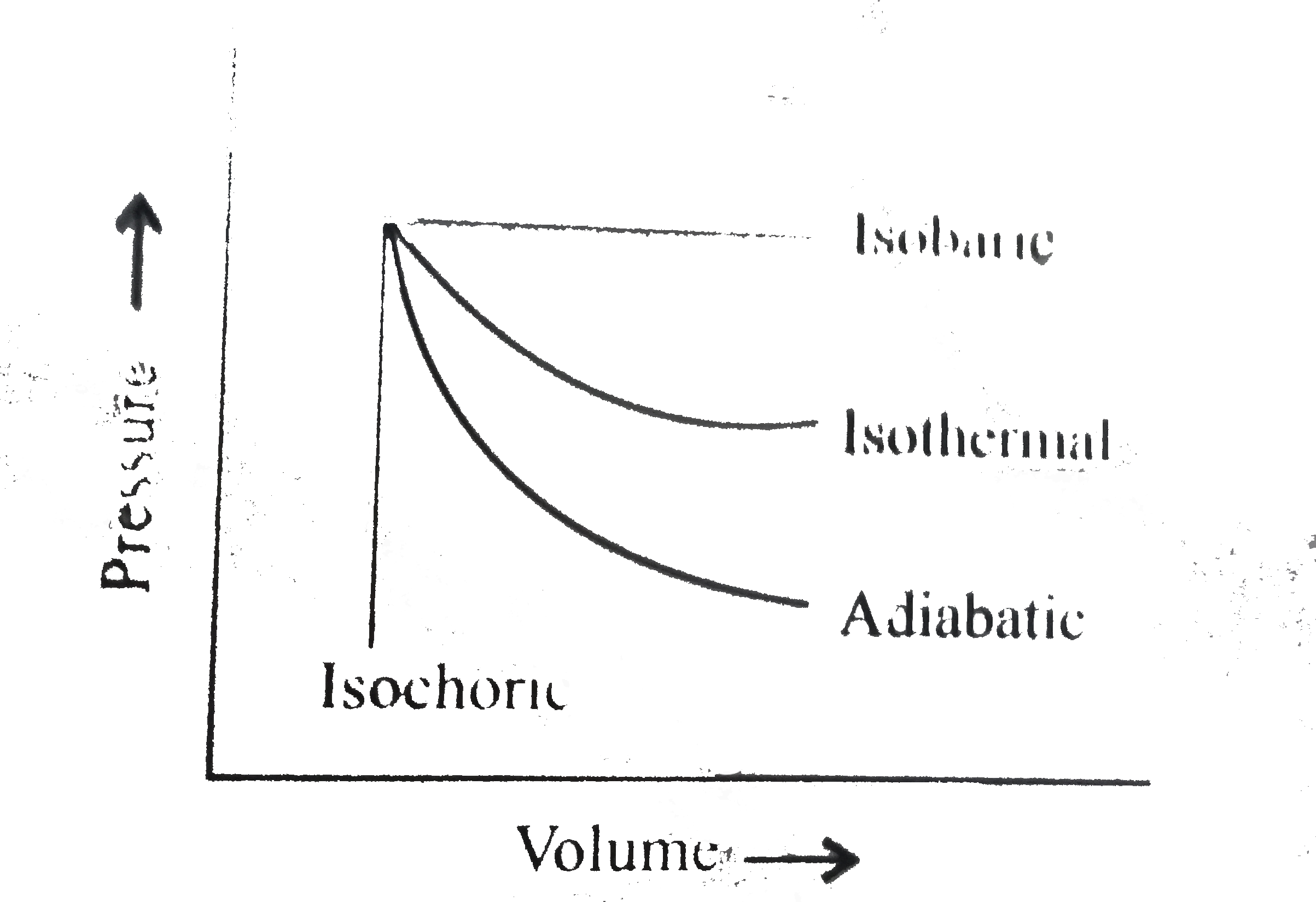
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- A cell is constructed by dipping a zinc rod in 0.1 M zinc nitrate solu...
Text Solution
|
- For the cell : Zn"|"Zn^(2+) (a=1)"||"Cu^(2+) (a=1)"|"Cu Given that ...
Text Solution
|
- Calculate the equivalent conductivity of 1M H(2)SO(4), whose specific...
Text Solution
|
- A current of 10 A is passed for 80 min and 27 seconds through a cell ...
Text Solution
|
- Calculate E("cell") at 25^(@)C for the reaction : Zn+Cu^(2+) (0.2...
Text Solution
|
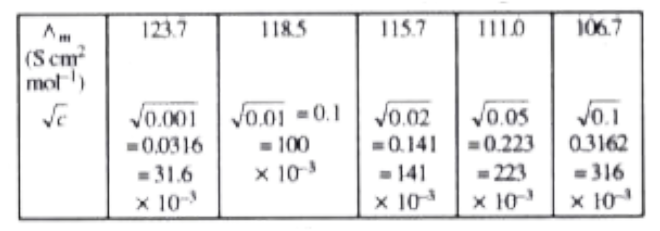
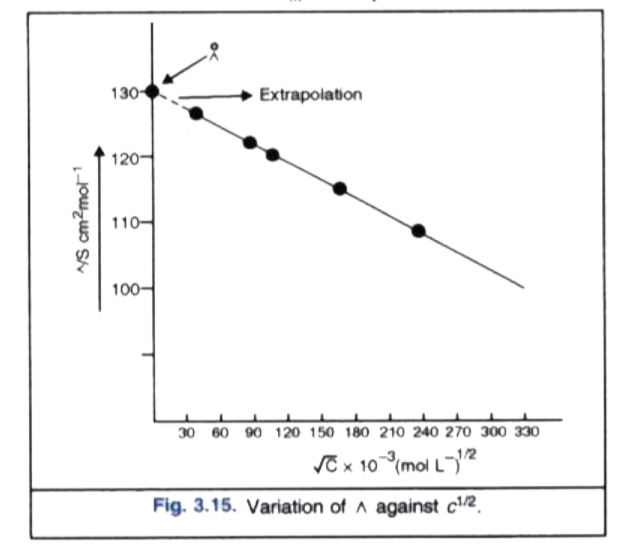
- A 0.05 M NaOH solution offered a resistance of 31.6 ohms in a conduct...
Text Solution
|
- For the following cell, calculate the emf: Al//Al^(3+) (0.01 M)"||"...
Text Solution
|
- A solution of 0.1 N KCl offers a resistance of 245 ohms. Calculate th...
Text Solution
|
- How many electrons will flow when a current of 5 amperes is passed thr...
Text Solution
|
- Consider the reaction 2Ag^(+) +Cd to 2Ag to 2Ag +Cd^(2+). The stan...
Text Solution
|
- Calculate the maximum work that can be obtained from the given electr...
Text Solution
|
- Calculate the value of E("cell") at 298 K for the following cell: ...
Text Solution
|
- 0*05 " M " NaOH solution offered a resistance of 31*6 ohm in a conduct...
Text Solution
|
- 0*3605 g of a metal is deposited on the electrode by passing 1*2 amper...
Text Solution
|
- How many hours does it take to reduce 3 moles of Fe^(3+) to Fe^(2+) wi...
Text Solution
|
- Calculate the emf of the following cell reaction at 298 K: Mg(s) +C...
Text Solution
|
- The specific conductance of a 0.01 M solution of acetic acid at 298 K...
Text Solution
|
- Calculate the number of coulombs required to deposit 5.4g of Al when ...
Text Solution
|
- A 0.05 M NH OH solution offers the resistance of 50 ohms to a conduct...
Text Solution
|