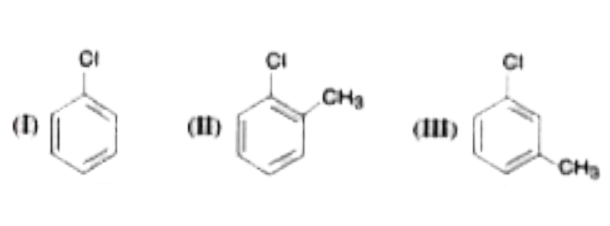
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
ICSE|Exercise EXERCISE (PART - I OBJECTIVE QUESTIONS) (CORRECT THE FOLLOWING STATEMENTS)|12 VideosHALOALKANES AND HALOARENES
ICSE|Exercise EXERCISE (PART - I OBJECTIVE QUESTIONS) |1 VideosHALOALKANES AND HALOARENES
ICSE|Exercise EXERCISE (PART - I OBJECTIVE QUESTIONS) (FILL IN THE BLANK)|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
ICSE|Exercise EXERCISE (PART-I ( OBJECTIVE QUESTIONS )) (Fill in the blanks)|60 VideosISC QUESTION PAPER
ICSE|Exercise PART-II(SECTION-B)|74 Videos
Similar Questions
Explore conceptually related problems
ICSE-HALOALKANES AND HALOARENES -EXERCISE (PART - I OBJECTIVE QUESTIONS) (MULTIPLE CHOICE QUESTIONS)
- The position of Br in the compound in CH(3)=CHC(Br)(CH(3))(2) can be c...
Text Solution
|
- Chlorobenzene is formed by reaction of chlorine with benzene in the pr...
Text Solution
|
- Ethylidene chloride is a/an…..
Text Solution
|
- What is 'A' in the following reaction ?
Text Solution
|
- A primary alkyl halide would prefer to undergo…….
Text Solution
|
- Which of the following alkyl halides will undergo S(N^1) reaction most...
Text Solution
|
- What is the correct IUPAC name for CH3-underset(C2H5)underset(|)"CH"...
Text Solution
|
- What should be the correct IUPAC name for diethylbromomethane?
Text Solution
|
- The reaction of toluene with chlorine in the presence of iron and in t...
Text Solution
|
- Chloromethane on treatment with excess of ammonia yields mainly
Text Solution
|
- Molecules whose mirror image is non-superimposable over them are known...
Text Solution
|
- Reaction of C6H5CH2Br with aqueous sodium hydroxide follows........
Text Solution
|
- Bond energies can be obtained by using the following relation: DeltaH ...
Text Solution
|
- Which of the following compounds will give racemic mixture on nucleoph...
Text Solution
|
- Arrange the compounds in increasing order of rate of reaction towards ...
Text Solution
|
- Arrange the compounds in increasing order of rate of reaction towards ...
Text Solution
|
- Arrange the compounds in increasing order of rate of reaction towards ...
Text Solution
|
- Arrange the compounds in increasing order of rate of reaction towards ...
Text Solution
|
- Which of the correct increasing order of boiling points of the followi...
Text Solution
|
- Which is the correct increasing order of boiling points of the followi...
Text Solution
|