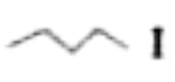
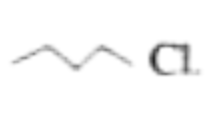Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
ICSE|Exercise EXERCISE (PART - II DESCRIPTIVE QUESTIONS) (SHORT ANSWER QUESTIONS)|94 VideosHALOALKANES AND HALOARENES
ICSE|Exercise EXERCISE (PART - II DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS)|65 VideosHALOALKANES AND HALOARENES
ICSE|Exercise EXERCISE (PART - I OBJECTIVE QUESTIONS) |1 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
ICSE|Exercise EXERCISE (PART-I ( OBJECTIVE QUESTIONS )) (Fill in the blanks)|60 VideosISC QUESTION PAPER
ICSE|Exercise PART-II(SECTION-B)|74 Videos
Similar Questions
Explore conceptually related problems
ICSE-HALOALKANES AND HALOARENES -EXERCISE (PART - II DESCRIPTIVE QUESTIONS) (VERY SHORT ANSWER QUESTIONS)
- Identify A and B in the CH3CH2 CH2Br overset("alc. KOH")to A overset(H...
Text Solution
|
- Identify A and B in the CH3Br + Mg ("ether") to A overset(H2O)toB.
Text Solution
|
- Give reasons: iodoform is obtained by the reaction of acetone with h...
Text Solution
|
- RCI is treated with Li in ether solution to form R-Li. R-Li reacts wit...
Text Solution
|
- Arrange the following compounds in order of increasing reactivity towa...
Text Solution
|
- In the following pair of halogen compounds which would undergo S(N^2) ...
Text Solution
|
- Arrange the following halides in order of increasing SN^(2) reactivit...
Text Solution
|
- Predict the order of reactivity of the following compounds in S(N^1) ...
Text Solution
|
- How will you distinguish between 1-bromopropane and allyl bromide ?
Text Solution
|
- Write a chemical reaction in which the iodide ion replaces the diazoni...
Text Solution
|
- In each of the following pairs of compounds, identify the compound whi...
Text Solution
|
- In each of the following pairs of compounds, identify the compound whi...
Text Solution
|
- (i)State one use each of DDT and iodoform. (ii) Which compound in t...
Text Solution
|
- Explain why in pair, (CH3)3C Cl " and " CH3Cl, CH3Cl will react faster...
Text Solution
|
- Which will have a higher boiling point : 1-Chloropentane or 2-Chloro...
Text Solution
|
- How will you convert methyl chloride into ethyl chloride ?
Text Solution
|
- Identify A and B in each of the following processes. CH3 CH2 Cl over...
Text Solution
|
- Assertion: Chloroform is stored in dark coloured bottles. Reason: Ch...
Text Solution
|
- State One use each of DDT and iodoform.
Text Solution
|
- How will you convert benzyl chloride to benzyl alcohol ?
Text Solution
|