Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PROPERTIES OF MATTER
ICSE|Exercise MODULE 3 (KINETIC THEORY OF GASES ) FROM KINETIC THEORY-rms SPEED , TEMPERATURE , PRESSURE|29 VideosPROPERTIES OF MATTER
ICSE|Exercise MODULE 4 ( TEMPERATURE ) CONCEPTUAL SHORT ANSWER QUESTIONS WITH ANSWERS|12 VideosPROPERTIES OF MATTER
ICSE|Exercise MODULE 3 (KINETIC THEORY OF GASES ) VERY SHORT ANSWER QUESTIONS|11 VideosOSCILLATIONS
ICSE|Exercise SELECTED PROBLEMS (OSCILLATION IN A TUNNEL BORED THROUGH THE EARTH)|2 VideosSAMPLE QUESTION PAPER - 01
ICSE|Exercise SECTION - D|12 Videos
Similar Questions
Explore conceptually related problems
ICSE-PROPERTIES OF MATTER-MODULE 3 (KINETIC THEORY OF GASES ) FROM GAS LAW AND EQUATION OF STATE
- An eletric bulb of volume 250 cm^3 was sealed off during manufacture a...
Text Solution
|
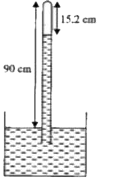
- The length of a faulty barometer is 90 cm and there is a little air ab...
Text Solution
|
- In a cubical vessel of side one metre a mixture of gases containing 22...
Text Solution
|
- Molar volume is the volume occupied by 1 mol of any ( ideal ) gas at s...
Text Solution
|
- An air bubble of volume 1.0cm^(3) rises from the cottom of a lake 40 m...
Text Solution
|
- A bulb contains air at atmospheric pressure at 40^(@)C temperature. Th...
Text Solution
|
- Estimate the total number of air molecules (inclusive of oxygen, nitro...
Text Solution
|
- The pressure inside an electric bulb is 10^(-3)mm of Hg at a temperatu...
Text Solution
|
- Two glass bulbs of same volume containing a gas at NTP are connected b...
Text Solution
|
- A vessel is filled with a gas at a pressure of 76 cm of Hg at some tem...
Text Solution
|
- 1.5 mole of N(2) at 77^(@)C and at a pressure of 5 atmosphere is mixed...
Text Solution
|
- A container 11g of a CO(2) and 7g of N(2) at 290K . What is the densit...
Text Solution
|