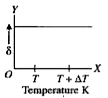
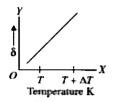
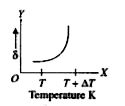
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
COMPETITION CARE UNIT
ICSE|Exercise INTERNAL ENERGY |40 VideosCOMPETITION CARE UNIT
ICSE|Exercise THERMAL CONDUCTION |17 VideosCOMPETITION CARE UNIT
ICSE|Exercise PROPERTIES OF MATTER (SURFACE TENSION ) |23 VideosCIRCULAR MOTION
ICSE|Exercise MODULE 2 (FROM ROTATIONAL KINETIC ENERGY , WORK ,POWER)|24 VideosDIMENSIONS
ICSE|Exercise SELECTED PROBLEMS (FROM CONVERSIONS OF ONE SYSTEMS OF UNITS INTO ANOTHER)|9 Videos
Similar Questions
Explore conceptually related problems
ICSE-COMPETITION CARE UNIT-PROPERTIES OF MATTER (CALORIMETRY, CHANGE OF STATE & KINETIC THEORY OF GASES )
- A lead ball moving with velocity v strikes a wall and stops. iF 50% of...
Text Solution
|
- if 1 g of system is mixed with 1 g of ice, then the resultant temperat...
Text Solution
|
- A gas at pressure p(0) is contained in a vessel. If the masses of all ...
Text Solution
|
- A sealed container with negligible thermal cofficient of expansion con...
Text Solution
|
- By what percentage should the pressure of a given mass of a gas be inc...
Text Solution
|
- The temperature of gas is produced by
Text Solution
|
- A polyatomic gas with n degrees of freedom has a mean energy per molec...
Text Solution
|
- If masses of all molecule of a gas are halved and their speed doubled ...
Text Solution
|
- The root mean square velocity of the gas molecule is 300 m/s. What wil...
Text Solution
|
- The mean translational K.E. per unit volume E and the pressure P of a ...
Text Solution
|
- A block of ice at -8^(@)C is slowly heated and converted to steam ...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. ITS volume is...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- If one mole of a monoatomic gas (gamma=5//3) is mixed with one mole of...
Text Solution
|
- The temperature of argon kept in a vessel is raised by 1^(@)C at a con...
Text Solution
|
- The temperature at which root mean square velocity of molecules of hel...
Text Solution
|
- At the same temperature the mean kinetic energies of molecular of hydr...
Text Solution
|
- For accuracy of gas thermometer, the gas should be filled at
Text Solution
|
- For measuring temperature near absolute zero, the thermometer used is
Text Solution
|
- Recently, the phenomenon of super conductivity has been observed at 95...
Text Solution
|