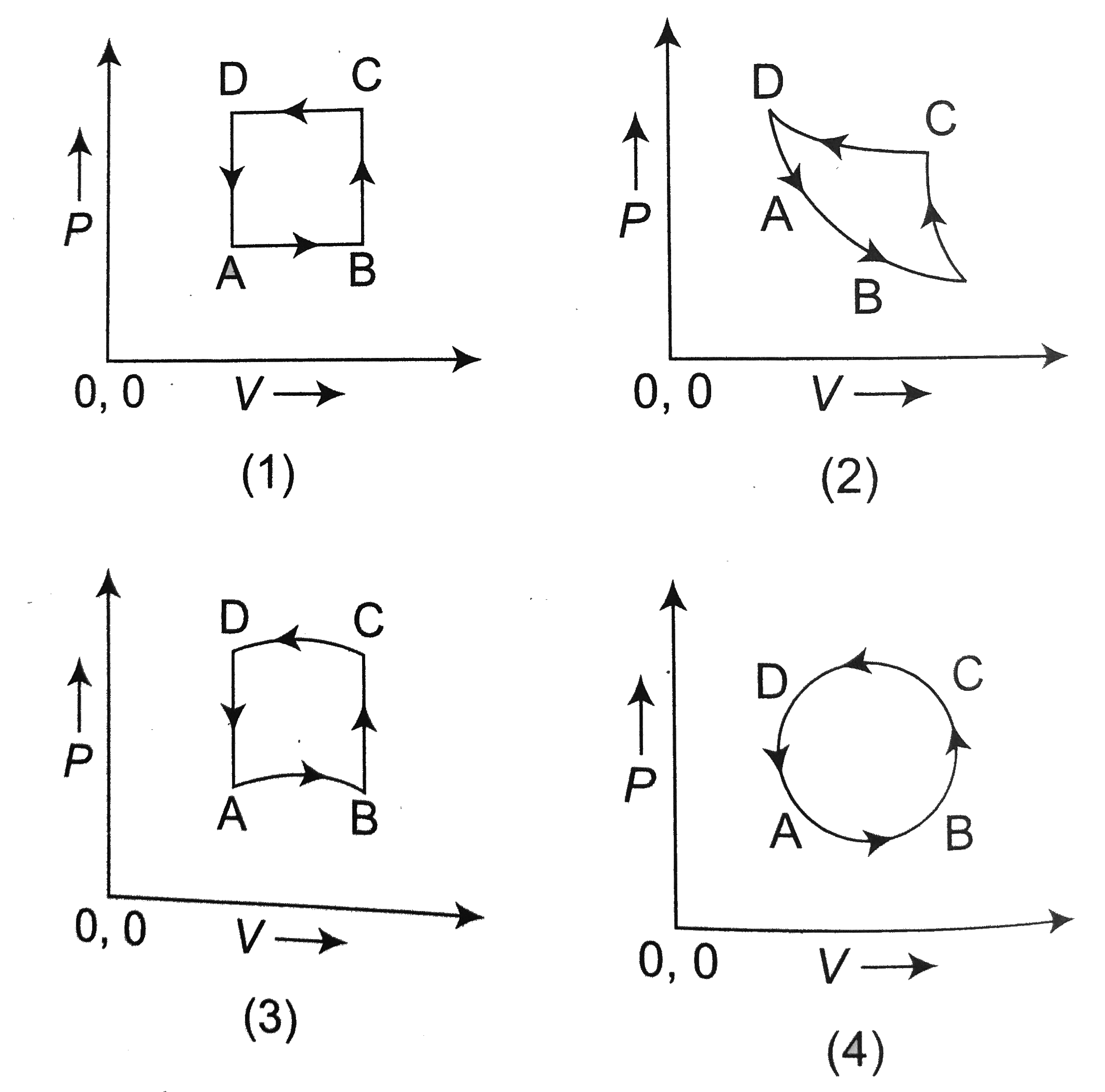
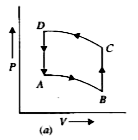
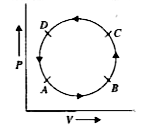
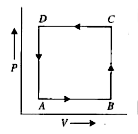
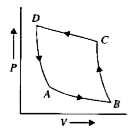
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COMPETITION CARE UNIT
ICSE|Exercise THERMAL CONDUCTION |17 VideosCOMPETITION CARE UNIT
ICSE|Exercise THERMAL RADIATION |13 VideosCOMPETITION CARE UNIT
ICSE|Exercise PROPERTIES OF MATTER (CALORIMETRY, CHANGE OF STATE & KINETIC THEORY OF GASES ) |30 VideosCIRCULAR MOTION
ICSE|Exercise MODULE 2 (FROM ROTATIONAL KINETIC ENERGY , WORK ,POWER)|24 VideosDIMENSIONS
ICSE|Exercise SELECTED PROBLEMS (FROM CONVERSIONS OF ONE SYSTEMS OF UNITS INTO ANOTHER)|9 Videos
Similar Questions
Explore conceptually related problems
ICSE-COMPETITION CARE UNIT-INTERNAL ENERGY
- A given system undergoes a change in which up workdone by the system e...
Text Solution
|
- The first law of thermodynamic is a special case of
Text Solution
|
- Choose the correct option: In following figs. Variation of volume by ...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- Which of the following is not a thermodynamic coordinate ?
Text Solution
|
- Heat energy absorbed by a system is going through a cyclic process sho...
Text Solution
|
- During an adiabatic expansion of 2 moles of a gas the chang in interna...
Text Solution
|
- A monatomic gas on n molecules is heated from temperature T(1) to T(2)...
Text Solution
|
- A Carnot engine operates with a source at 500 K and sink at 375 K. Eng...
Text Solution
|
- If R is universal gas constant , the amount of heat needed to raise th...
Text Solution
|
- A frictionless heat engine can be 100% efficient only if its exhaust t...
Text Solution
|
- The temperateus of inside and outside of a refrigerator are 273 K and ...
Text Solution
|
- An ideal gas heat engine operates in Carnot cycle between 227^(@)C and...
Text Solution
|
- 1 cm^(3) of waterr at its boiling point absorbs 540 calories of heat t...
Text Solution
|
- If a refrigerator's door is kept open, will the room become cool or ho...
Text Solution
|
- A Carnot engine has an efficiency of 1//6. When the temperature of the...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- A gas mixture coinsists of (2) moles of oxygen and (4) moles of argon ...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- An ideal gas is taken through cycle A to B to C-A, as shown in Fig. IF...
Text Solution
|