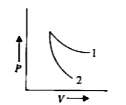
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COMPETITION CARE UNIT
ICSE|Exercise THERMAL CONDUCTION |17 VideosCOMPETITION CARE UNIT
ICSE|Exercise THERMAL RADIATION |13 VideosCOMPETITION CARE UNIT
ICSE|Exercise PROPERTIES OF MATTER (CALORIMETRY, CHANGE OF STATE & KINETIC THEORY OF GASES ) |30 VideosCIRCULAR MOTION
ICSE|Exercise MODULE 2 (FROM ROTATIONAL KINETIC ENERGY , WORK ,POWER)|24 VideosDIMENSIONS
ICSE|Exercise SELECTED PROBLEMS (FROM CONVERSIONS OF ONE SYSTEMS OF UNITS INTO ANOTHER)|9 Videos
Similar Questions
Explore conceptually related problems
ICSE-COMPETITION CARE UNIT-INTERNAL ENERGY
- A gas mixture coinsists of (2) moles of oxygen and (4) moles of argon ...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- An ideal gas is taken through cycle A to B to C-A, as shown in Fig. IF...
Text Solution
|
- In a given process on an ideal gas, dW = 0 and dQ lt 0. Then for the g...
Text Solution
|
- When a gas expands adiabatically
Text Solution
|
- The specific heat of a gas in an isothermal process is
Text Solution
|
- When the system does not exchange heat with the surroundings, the proc...
Text Solution
|
- A diatomic gas initially at 18^(@)C is compressed adiabatically to one...
Text Solution
|
- For a gas gamma = 5//3 and 800 c.c, of this gas is suddenly compressed...
Text Solution
|
- The pressure inside a tyre is 4 times that of atmosphere. If the tyre ...
Text Solution
|
- The gas law (PV/T) = constant is true for
Text Solution
|
- Two identical samples of a gas are allowed to expand (i) isothermally ...
Text Solution
|
- Certain perfect gas is found to obey pV^(3//2) = constant during adi...
Text Solution
|
- Two samples, A and B of a gas at the same initial temperature and pres...
Text Solution
|
- A mass of ideal gas at pressure P is expanded isothermally to four tim...
Text Solution
|
- Starting from the same initial conditions, an ideal gas expands from v...
Text Solution
|
- A monatomic ideal gas, initially at temperature T(1) is enclosed in a ...
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in Fig. 1...
Text Solution
|
- In an adiabatic change the pressure and temperature of monoatomic gas ...
Text Solution
|
- Slope of PV and V for an isobaric process will be
Text Solution
|