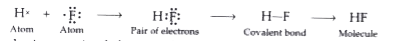Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING
ICSE|Exercise ADDITIONAL QUESTION|24 VideosCHEMICAL BONDING
ICSE|Exercise UNIT TEST PAPER 2|17 VideosCHEMICAL BONDING
ICSE|Exercise Questions from Previous ICSE Board Papers (2018)|6 VideosANALYTICAL CHEMISTRY-USE OF AMMONIUM & SODIUM HYDROXIDE
ICSE|Exercise Additional Questions|6 VideosCHEMISTRY 2011
ICSE|Exercise SECTION-II|48 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL BONDING -QUESTIONS
- Elements Q and S react together to form an ionic compound. Under norm...
Text Solution
|
- Can Q and S, both be metals ? Justify your answer.
Text Solution
|
- Atomic number of hydrogen is 1 and the atomic number of fluorine atom ...
Text Solution
|
- State the type of bonding in the following molecules - [i] Water , [...
Text Solution
|
- Predict the type of bonding in the following molecules: calcium ox...
Text Solution
|
- Describe the formation of a double bond in a molecule of carbon dioxid...
Text Solution
|
- Write the valency of each atom in CO2
Text Solution
|
- Draw an electron dot diagram to show the formation of each of the foll...
Text Solution
|
- Draw an electron dot diagram to show the formation of each of the foll...
Text Solution
|
- Hydrogen chloride can be termed as a polar covalent compound. Give rea...
Text Solution
|
- An element L consists of molecules. What type of bonding is involved...
Text Solution
|
- An element L consists of molecules. When L is heated with iron metal...
Text Solution
|
- Choose the correct answer from the options given below: Which of th...
Text Solution
|
- The following table shows the electronic configurations of the element...
Text Solution
|
- The following table shows the electronic configurations of the element...
Text Solution
|
- The following table shows the electronic configurations of the element...
Text Solution
|
- The following table shows the electronic configurations of the element...
Text Solution
|
- Compound X consists of molecules. Choose the letter corresponding to ...
Text Solution
|
- Compound X consists of molecules. Choose the letter corresponding t...
Text Solution
|
- Compound X consists of molecules. Choose the letter corresponding to ...
Text Solution
|