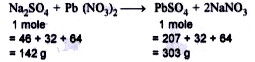
Text Solution
Verified by Experts
Topper's Solved these Questions
MOLE CONCEPT AND STOICHIOMETRY
ICSE|Exercise WORKSHEET-1|10 VideosMOLE CONCEPT AND STOICHIOMETRY
ICSE|Exercise WORKSHEET-2|37 VideosMOLE CONCEPT AND STOICHIOMETRY
ICSE|Exercise ILLUSTRATIVE ASSIGNMENTS|28 VideosORGANIC CHEMISTRY
ICSE|Exercise QUESTIONS FOR PRACTICE PAPER ON EXAMINATION PATTERN (ANSWER THE QUESTIONS)|40 Videos
Similar Questions
Explore conceptually related problems