Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 3-QUESTION
- Complete the following chemical equations : (i) Cr2O6^(2-)+6Fe^(2+)...
Text Solution
|
- Complete the reaction: 2MnO(4)^(-)+5C(2)O(4)^(2-) +16H^(+) to
Text Solution
|
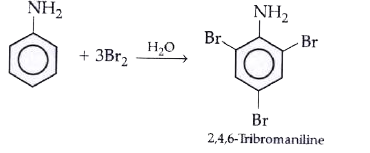
- Write balanced equations of the following reactions: Aniline and Br...
Text Solution
|
- Write balanced equations of the following reactions: Ethylamine and...
Text Solution
|
- Write balanced equations of the reaction: Acetic anhydride and ammon...
Text Solution
|
- (i) Name the element of 3d transition series which shows maximum numbe...
Text Solution
|
- Out of Cr^(3+) and Mn^(3+) , which is a stronger oxidising agent and ...
Text Solution
|
- Explain giving reasons: (i) Transition metals and many of their compou...
Text Solution
|
- Name the method used for the refining of Nickel metal.
Text Solution
|
- (a) Give an example of zone refining of metals. (b) What is the role ...
Text Solution
|
- What is the role of limestone in the extraction of iron from its oxide...
Text Solution
|
- Calculate E("cell") at 25^(@)C for the reaction : Zn+Cu^(2+) (0.2...
Text Solution
|
- For the following cell, calculate the emf: Al//Al^(3+) (0.01 M)"||"...
Text Solution
|
- A reaction is first order in A and second order in B (i) Write the d...
Text Solution
|
- A reaction is first order in A and second order in B (i) Write the d...
Text Solution
|
- A reaction is first order in A and second order in B (i) Write the d...
Text Solution
|
- (a) Explain the following terms : (i) Order of a reaction (ii) Mol...
Text Solution
|
- (a) Draw the structures of the following : (i) PCl(5)(s) (ii) ...
Text Solution
|
- Account for the follwing : (i) Bi(v) is a stronger oxidizing agent ...
Text Solution
|
- Account for the follwing : (i) Bi(v) is a stronger oxidizing agent ...
Text Solution
|