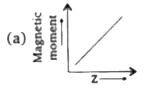
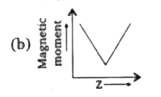
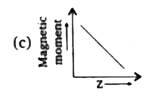
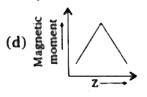
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise Level 3 (Passage Type)|89 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWERS IN/ARE CORRECT|98 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|4 Videos
Similar Questions
Explore conceptually related problems