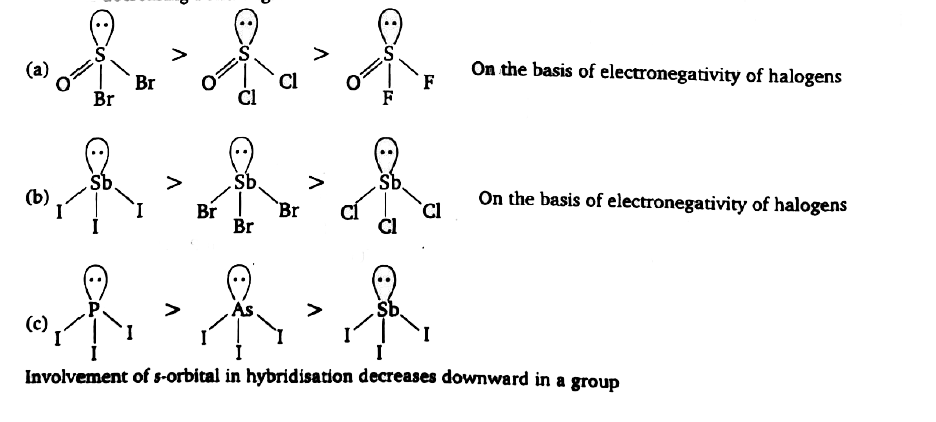
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise Level 3 (Passive 1)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise Level 3 (Passive 2)|6 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|54 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|64 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL ENGLISH|Exercise LEVEL 2|144 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (BASIC)-Level 2
- Incorrect statement is :
Text Solution
|
- Which of the following set contains species having same angle around t...
Text Solution
|
- Which of the following compound has the smallest (X-A-X) bond angle in...
Text Solution
|
- The incorrect order of boiling point is :
Text Solution
|
- Iodine molecules are held in the solid lattice by
Text Solution
|
- Carbon dioxide is a gas but silica is a solid because:
Text Solution
|
- Choose the correct code of characteristics for the given order of hybr...
Text Solution
|
- Which is correct statement ? As the s-character of a hybrid orbital ...
Text Solution
|
- Which of the following is incorrectly matched ?
Text Solution
|
- The ionic bond X^(+)Y^(-) are formed when : (I) electron affinity of...
Text Solution
|
- In the Born-Haber cycle for the formation of solid common salt (NaCl),...
Text Solution
|
- Species having maximum 'Cl-O' bond order is :
Text Solution
|
- Which of the following species contains minimum number of atoms in XY ...
Text Solution
|
- The molecule ML(x) is planar with 7 pairs of electrons around M in the...
Text Solution
|
- Choose the correct option for the collowing molecule in view of chemic...
Text Solution
|
- Which of the following statement is correct about I(3)^(+) and I(3)^(-...
Text Solution
|
- In which of the following molecular shape d(z^(2)) orbital must not be...
Text Solution
|
- The correct statement regarding SO(2) molecule is :
Text Solution
|
- A molecule XY(2) contains two sigma bonds two pi bond and one lone pai...
Text Solution
|
- In which of the following pairs, both the species have the same hybrid...
Text Solution
|