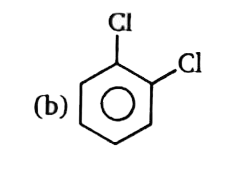
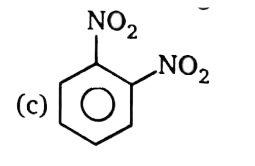
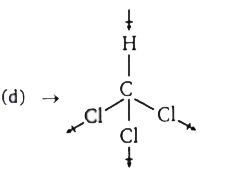
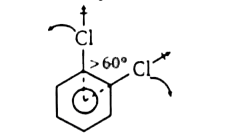
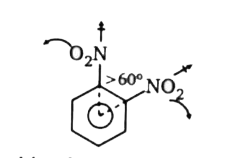
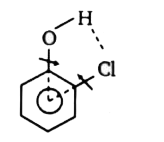
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise Level 3|77 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|78 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|64 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|54 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-Level 2
- The geometry with respect ot the central atom of the following molecul...
Text Solution
|
- Incorrect statement regarding BF(3)NH(3) molecule is :
Text Solution
|
- In which of the following molecules mu(oberved) is found to be greater...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Which of the following compounds has dipole moment approximately equal...
Text Solution
|
- Which of the following bonds have lowest bond energy?
Text Solution
|
- The bond having the minimum bond energy is :
Text Solution
|
- Arrange in increasing order of extent of hydrolysis [C Cl(4),MgCl(2),A...
Text Solution
|
- Inorganic benzene reacts with HCl to form a compounds B(3)N(3)H(9)Cl(...
Text Solution
|
- Select the correct statements about hydrolysis of BCl(3) and NCl(3):
Text Solution
|
- The incorrect statement regarding molecular orbital (s) is :
Text Solution
|
- Which of the following species absorb maximum energy in its HOMO-LUMO ...
Text Solution
|
- If P to T are second period p-block elements then which of the followi...
Text Solution
|
- Which of the following facts given is not correct? (I) Bond length o...
Text Solution
|
- N(2) and O(2) are converted to monocations N(2)^(+) and O(2)^(+) respe...
Text Solution
|
- In which of the following transformations, the bond order has increase...
Text Solution
|
- H.O.M.O (Highest Occupied Molecular Orbital ) of CO molecular is :
Text Solution
|
- The structure of B(3)N(3)H(6) is as follows: How may derivations...
Text Solution
|
- Correctly match is:
Text Solution
|
- Select correct statement (s) :
Text Solution
|