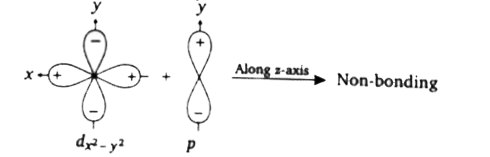A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-ASSERTION-REASON TYPE QUESTIONS
- Assertion: Superoxides of alkali metals are paramagnetic. Reason: Su...
Text Solution
|
- Assertion : The HF(2)^(-) ion exists in the solid state & also in liqu...
Text Solution
|
- Assertion: If d(x^(2)-y^(2)) and p(y) orbitals come close together al...
Text Solution
|
- Assertion : The H-bond present in NH(3) dissolved in water is best rep...
Text Solution
|
- Assertion : C(3)O(2) is non-polar molecule. Reason : Terminal pi-bon...
Text Solution
|
- Assertion : If d(x^(2)-y^(2)) and p(y) orbitals come close together al...
Text Solution
|
- Assertion : BF(3) undergoes in partial hydrolysis Reason : Due to st...
Text Solution
|
- Assertion : The central catbon atom in F(2)C=C=CF(2) and both carbo at...
Text Solution
|
- Assertion : Formation of PH(4)^(+) ion is relatively difficult in comp...
Text Solution
|
- Assertion : Bond dissociation energy of B-F bond in BF(3) molecule is ...
Text Solution
|
- Assertion : PF(3) is stronger lewis base than PH(3). Reason : l.p. o...
Text Solution
|
- Assertion :NiO is less than CaO. Reason :Ni^(2+) is pseudo noble gas...
Text Solution
|
- Assertion : When two gaseous OF molecules are allowed to cool, than th...
Text Solution
|
- Assertion : bond dissociation energy of N-F bond in NF(3) molecule is ...
Text Solution
|
- Assertion: If d(x^(2)-y^(2)) and p(y) orbitals come close together al...
Text Solution
|
- Assertion : H(2)S is stronger acid than PH(3). Reason : S is more el...
Text Solution
|
- Assertion : NaCl is more ionic than NaI. Reason : Chlorine is more e...
Text Solution
|
- Assertion : PbI(4) doesn't exist and converts into PbI(2) and I(2) spo...
Text Solution
|
- Assertion : Dipole moment of NF(3) is less than that of NH(3). Reaso...
Text Solution
|
- Assertion : Solubility of n-orbital of n-orbital in water decrease w...
Text Solution
|