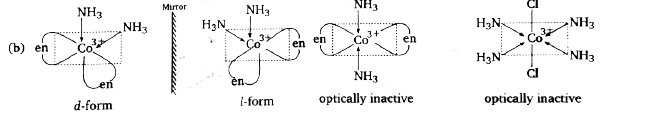
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CO-ORDINATION COMPOUNDS-LEVEL 2
- Which complex is likely to show optical activity :
Text Solution
|
- Which of the following statement is true?
Text Solution
|
- The following complexs are given? trans-[Co(NH(3))(4)1(2)]^(o+) ci...
Text Solution
|
- Which of the following can show geometrical isomerism?
Text Solution
|
- Which of the following compounds will exhibit cis-trans (geometrical) ...
Text Solution
|
- Which of the following will have two stereoisomeric forms? (I) [Cr(N...
Text Solution
|
- A coordination complex of type MX(2)Y(2) (M-metal ion: X, Y-monodentat...
Text Solution
|
- The ratio of cis and trans-isomers of the complex [Ma(2)bcde]^(n+-) is...
Text Solution
|
- [PdCl(2)(PM e(3))(2)] is a diamagnetic complex of Pd (II). How many un...
Text Solution
|
- Complex compounds(s) which is optical active and does ot dpeend upon t...
Text Solution
|
- Choose the correct code regarding, possible number of geometrical isom...
Text Solution
|
- How many geometrical isomers are possible for complex [Mab(AB)(2)]^(n+...
Text Solution
|
- [CoCl(2)(NH(3))(4)]^(+)+Cl^(-) to [CoCl(3)(NH(3))(3)]+NH(3). If in thi...
Text Solution
|
- Select the correct statement about given square planar complex.
Text Solution
|
- Select the correct code regarding total number of space isomers for th...
Text Solution
|
- How many geometrical isomers are possible for [Pd^(2+)(NH(2)-CH(CH(3))...
Text Solution
|
- How many geometrical isomers are possible [Co(acac)(2)BrCl]^(Theta) ar...
Text Solution
|
- Which of the following compounds will exhibit geometrical isomerism ?
Text Solution
|
- In which case racemic mixture is obtained on mixing its mirror images ...
Text Solution
|
- Which of the following compound show optical isomerism?
Text Solution
|