A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
p-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise LEVEL 3 (PASSAGE TYPE)|17 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWERS IS/ARE CORRECT|95 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosMETALLURGY
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|29 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-p-BLOCK ELEMENTS-LEVEL 2
- Anhydrous AlCl(3) is covalent however, when it is dissolved in water h...
Text Solution
|
- Borax in its crystal posses:
Text Solution
|
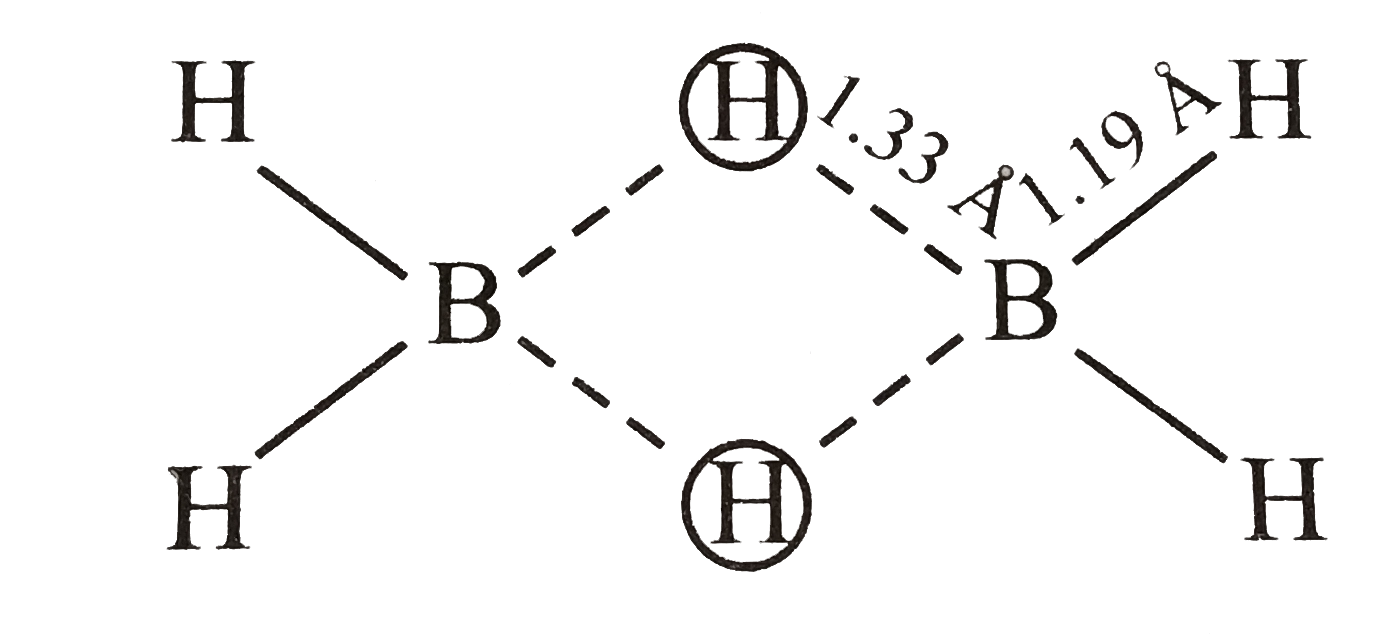
- The molecular shapes of diborane is shown below : . Consider the f...
Text Solution
|
- Alumiium vessels should not be washed with materials containing washin...
Text Solution
|
- Which of the following statements about anhydrous aluminium chloride i...
Text Solution
|
- Na(2)B(4)O(7).10H(2)Ooverset("Heat")rarrX + NaBO(2) + H(2)O, X + Cr(2)...
Text Solution
|
- Borax is converted into amorphous Boron by following steps "Borax"ov...
Text Solution
|
- The dissolution of Al(OH)(3) by a solution of NaOH results in the form...
Text Solution
|
- Choose the correct sequence for the geometry of the given molecules ...
Text Solution
|
- What is not true about borax ?
Text Solution
|
- How can the following reaction be made to proceed in forward direction...
Text Solution
|
- Which of the following catio can not give bead test?
Text Solution
|
- The incorrect statement regarding above reactions is:
Text Solution
|
- The incorrect statement regarding 'X' in given reaction is: BF(3)+Li...
Text Solution
|
- The incorrect stability order of +3 and +1 states of 13th group elemen...
Text Solution
|
- Consider the following route of reaction: R(2)SiCl(2)+"water" to (A)...
Text Solution
|
- The most basic oxide of elements group 14 of the periodic table is:
Text Solution
|
- (Si(2)O(5))(n)^(2n-) anion is obtained when:
Text Solution
|
- Amphilbole silicate structure has 'x' number of corner shared per tetr...
Text Solution
|
- The silicate ion in the mineral kinoite is a chain of three SiO(4)^(4-...
Text Solution
|
 .
.