A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
p-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise LEVEL 3 (PASSAGE TYPE)|17 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWERS IS/ARE CORRECT|95 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosMETALLURGY
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|29 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videos
VK JAISWAL ENGLISH-p-BLOCK ELEMENTS-LEVEL 2
- The correct order of S-S bond length in following oxyanions is: S(2)...
Text Solution
|
- In which of the following reaction product does not contian 'peroxoy' ...
Text Solution
|
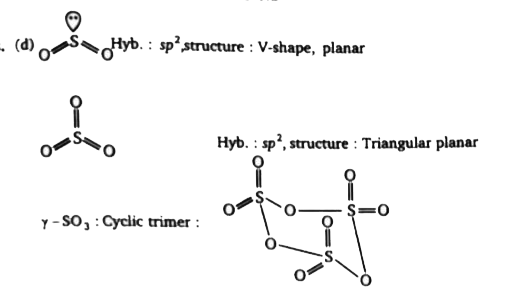
- Consider the following statements in respect of oxides of sulphur: (...
Text Solution
|
- Gas that can not be collected over water is:
Text Solution
|
- In thiosulphuric acid:
Text Solution
|
- One gas bleaches the colour of flowers by reduction, while the other b...
Text Solution
|
- Which of the following halides cannot be hydrolysed at room temperatur...
Text Solution
|
- By which of the following methods, H(2)O(2) can't be synthesised?
Text Solution
|
- Give the correct order of initials T or F for following statements. Us...
Text Solution
|
- Which of the following parent acid(s) does/do not have corresponding h...
Text Solution
|
- Which pair of elements cann from multiple bond with itselff and oxygen...
Text Solution
|
- Consider the following reactions: (i) PCl(3)+3H(2)O to H(3)PO(3) +3H...
Text Solution
|
- Consider the oxy acids HClOn series here value of n is 1 to 4. then i...
Text Solution
|
- The correct statement regarding ClO(n)^(-) molecular ion is:
Text Solution
|
- In, Cl(2)O(6)(l)+HF to P+Q If H^(-) of acid HF attraches with Q, the...
Text Solution
|
- Bromine is commercially prepared from sea water by displacement reacti...
Text Solution
|
- Which of the following properties of halogens increase with increasing...
Text Solution
|
- Predict the correct product when Cl(2) passed through H-overset(18)(O)...
Text Solution
|
- Cl(2)(g)+Ba(OH)(2) to X(aq.)+BaCl(2)+H(2)O X+H(2)SO(4) to Y+BaSO(4) ...
Text Solution
|
- Auto-oxidation of bleaching powder gives:
Text Solution
|