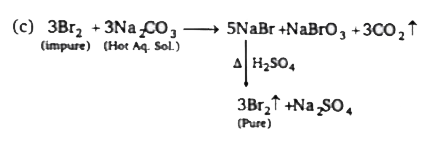A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
p-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise LEVEL 3 (PASSAGE TYPE)|17 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWERS IS/ARE CORRECT|95 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosMETALLURGY
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|29 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-p-BLOCK ELEMENTS-LEVEL 2
- The correct statement regarding ClO(n)^(-) molecular ion is:
Text Solution
|
- In, Cl(2)O(6)(l)+HF to P+Q If H^(-) of acid HF attraches with Q, the...
Text Solution
|
- Bromine is commercially prepared from sea water by displacement reacti...
Text Solution
|
- Which of the following properties of halogens increase with increasing...
Text Solution
|
- Predict the correct product when Cl(2) passed through H-overset(18)(O)...
Text Solution
|
- Cl(2)(g)+Ba(OH)(2) to X(aq.)+BaCl(2)+H(2)O X+H(2)SO(4) to Y+BaSO(4) ...
Text Solution
|
- Auto-oxidation of bleaching powder gives:
Text Solution
|
- Which is incorrectly matched?
Text Solution
|
- The three elements X,Y and Z with electronic configuration shown below...
Text Solution
|
- The incorrect order is:
Text Solution
|
- The correct statement regarding perxenate ion (XeO(6)^(4-)) is:
Text Solution
|
- XeF(2) and XeF(6) are separately hydrolysed then:
Text Solution
|
- Mf + XeF(4) rarr M^(+) A^(-) (M^(+)- alkali metal cation) The state of...
Text Solution
|
- Xenon tetrafluoride, XeF(4) is:
Text Solution
|
- XeF(6) dissolves in anhydrous HF to give a good conducting solution wh...
Text Solution
|
- Which of the following is not true about helium ?
Text Solution
|
- SbF(5) reacts with XeF(4) to form an adduct. The shapes of cation and ...
Text Solution
|
- Consider the followingg transformations: (I) XeF(6)+NaF to Na^(+)[Xe...
Text Solution
|
- Which of the following is an uncommon hydrolysis product of XeF(2) and...
Text Solution
|
- Incorrect statement regarding following reaction is:
Text Solution
|