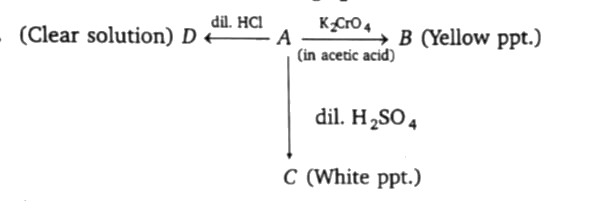
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise LEVEL 3|62 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|43 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|4 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videoss-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|2 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-QUALITATIVE INORGANIC ANALYSIS-LEVEL 2
- SO(3)^(2-)+S^(***) overset("boil")to S S^(***)O(3)^(2-),S S^(***)O(3)^...
Text Solution
|
- The sequential unknown reagents is/are:
Text Solution
|
- Compound(s) is/are:
Text Solution
|
- Which of the following is presipitated when an arsenate reacts withh a...
Text Solution
|
- A coloured solution known to contain two metal ions, was treated with ...
Text Solution
|
- Which of the following statement is incorrect? (I) In S(2)O(3)^(2-) ...
Text Solution
|
- On strongly heating, a blue salt leaves a black residue. Which of the ...
Text Solution
|
- Which of the following, when dissolved in yellow ammonium sulphide, fo...
Text Solution
|
- Thenard's blue is
Text Solution
|
- A salt imparts a yellow colour to a borax bead in an oxidising flame. ...
Text Solution
|
- BiCl(3) can be reduced to metallic bismuth by:
Text Solution
|
- The blue colour in an oxidising flame of a microcosmic bead containing...
Text Solution
|
- Which of the following reaction(s) relevat t the microcosmic salt bead...
Text Solution
|
- Which of the following is formed in solution when [Cu(NH(3))(4)]^(2+) ...
Text Solution
|
- A white solid forms Rinmann's greenn in the charcoal cavity test in an...
Text Solution
|
- A white solid imparts a violet colour to a Bunsen flame. On being heat...
Text Solution
|
- Which of the following is soluble in boiling water, but less soluble i...
Text Solution
|
- Which of the following pairs of cations cannot be separated by using d...
Text Solution
|
- If NH(4)OH in presence of NH(4)Cl is added to a solution containing Al...
Text Solution
|
- Which of the following pairs of cations ca be separated by adding NH(4...
Text Solution
|