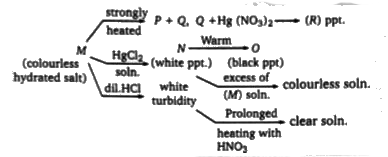
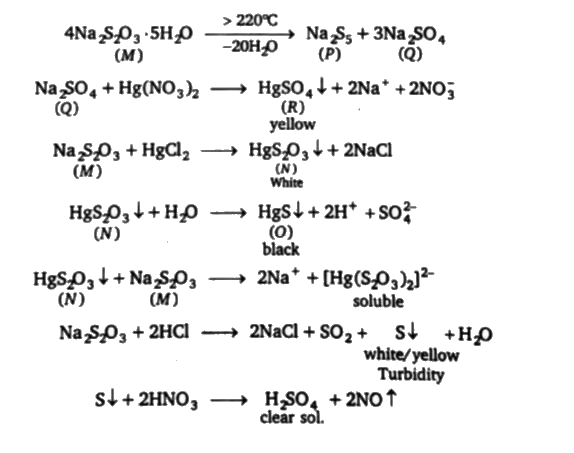
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|43 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|4 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise LEVEL 2|76 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videoss-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|2 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-QUALITATIVE INORGANIC ANALYSIS-LEVEL 3
- Q. The colour of the compound R is:
Text Solution
|
- Q. The structure of compound P is:
Text Solution
|
- Q. Compound M is used (I) In photography (II) ini analytical chemi...
Text Solution
|
- A white crystalline solid 'A' on boiling with caustic soda solution gi...
Text Solution
|
- A white crystalline solid 'A' on boiling with caustic soda solution gi...
Text Solution
|
- A white crystalline solid 'A' on boiling with caustic soda solution gi...
Text Solution
|
- A chemist opened a cupboard to find four bottles containing water solu...
Text Solution
|
- A chemist opened a cupboard to find four bottles containing water solu...
Text Solution
|
- A chemist opened a cupboard to find four bottles containing water solu...
Text Solution
|
- A chemist opened a cupboard to find four bottles containing water solu...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- A coloured compound (A) reacts with dilute H(2)SO(4) to produce a colo...
Text Solution
|
- Q. The structure of compound (A) is:
Text Solution
|
- Read the following short write up and answer subsequent questions base...
Text Solution
|
- An unknown mixture contains one or two of the following: CaCO(3),BaCl(...
Text Solution
|
- An unknown mixture contains one or two of the following: CaCO(3),BaCl(...
Text Solution
|
- An unknown mixture contains one or two of the following: CaCO(3),BaCl(...
Text Solution
|