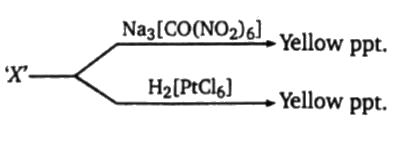A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|4 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|14 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise LEVEL 3|62 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videoss-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|2 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-QUALITATIVE INORGANIC ANALYSIS-ONE OR MORE ANSWER IS/ARE CORRECT
- When ozone reacts with an excess of potassium iodide solution buffered...
Text Solution
|
- Which of the following pairs of cations cannot be separate by using an...
Text Solution
|
- Aq. Solution of the cation(s) present in 'X' is/are:
Text Solution
|
- Potassium chromate solution is added to an aqueous solution of a metal...
Text Solution
|
- A white sublimable solid, when boiled with an NaOH solution, gives a c...
Text Solution
|
- Which of the following option(s) is/are correct reagarding 'P' among t...
Text Solution
|
- In which of the following case a violet colouration be observed?
Text Solution
|
- Saturated solution of SO(2) is heated at 150^(@)C in a closed containe...
Text Solution
|
- Which reaction is/are possible?
Text Solution
|
- Which of the following combinations in an aqueous medium will give a b...
Text Solution
|
- Which of the following statements (s) is (are) correct with reference ...
Text Solution
|
- Which of the following combinations in an aqueous medium will give a r...
Text Solution
|
- Which of the following sulphates are soluble in water?
Text Solution
|
- Which of the following pair(s) contain species, which react with each ...
Text Solution
|
- Which of the following sulphides dissolve in dilute HCl?
Text Solution
|
- Acidic K(2)Cr(2)O(7) reacts with H(2)S to produce:
Text Solution
|
- Which of the following reagents can be used to distinguish between SO(...
Text Solution
|
- Which of the followng will dissolve in a mixuture of NaOH and H(2)O(2)...
Text Solution
|
- Which of the following reagents willnot be useful in separating a mixt...
Text Solution
|
- Which of the following mixtures cannot be separated by passing H(2)S t...
Text Solution
|