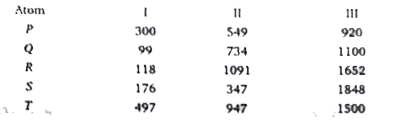A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWERS IN/ARE CORRECT|98 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCHTHE COLUMN|11 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|4 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-PERIODIC PROPERTIES-Level 3 (Passage Type)
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- The I.E(1). And the I.E(2) in kJ mol^(-1) of a few elements designated...
Text Solution
|
- The I.E(1). And the I.E(2) in kJ mol^(-1) of a few elements designated...
Text Solution
|
- The I.E(1). And the I.E(2) in kJ mol^(-1) of a few elements designated...
Text Solution
|
- The I.E(1). And the I.E(2) in kJ mol^(-1) of a few elements designated...
Text Solution
|
- Elements with their electronic configuration are given below: Answer...
Text Solution
|
- Elements with their electronic configuration are given below: Answer...
Text Solution
|
- Elements with their electronic configuration are given below: Answer...
Text Solution
|
- Elements with their electronic configuration are given below: Answer...
Text Solution
|
- J.C. Slater proposed an empirical constant that represents the cumulat...
Text Solution
|
- J.C. Slater proposed an empirical constant that represents the cumulat...
Text Solution
|
- J.C. Slater proposed an empirical constant that represents the cumulat...
Text Solution
|
- The electronegativity of the following elements increases in the order...
Text Solution
|
- Metals have few electrons in their valence shell while non-metals gene...
Text Solution
|
- The value of four quantum number for the last electron of atom of elem...
Text Solution
|