Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCHTHE COLUMN|11 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|36 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise Level 3 (Passage Type)|89 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|4 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-PERIODIC PROPERTIES-ONE OR MORE ANSWERS IN/ARE CORRECT
- In halogens, which of the following decreases from fluorine to iodine?
Text Solution
|
- Mark the correct statements out of the following:
Text Solution
|
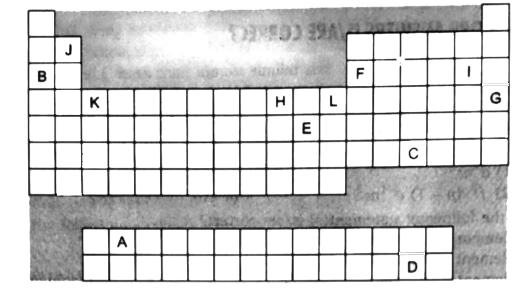
- The diagram below shows part of the skeleton of the periodic table ini...
Text Solution
|
- Answer the following on the basis of modern periodic table. (I) G...
Text Solution
|
- Which of the following statements concerning elements with atomic numb...
Text Solution
|
- Which of the following pairs of elements have same number of electrons...
Text Solution
|
- A change of Zn to Zn^(2+) is a accompanied by a decrease in:
Text Solution
|
- Which elements is named after the name of a planet is
Text Solution
|
- The properties which are common to both groups 1 and 17 elements in th...
Text Solution
|
- There are three elements A, B and C. their atomic number are Z(1), Z(2...
Text Solution
|
- Consider the following representation based on long form of periodic t...
Text Solution
|
- Which of the following match is/are correct regarding B, Al, C and S e...
Text Solution
|
- Consider the value of all four quantum number for last electron and sp...
Text Solution
|
- An element 'X' present in its ground state, the value of principal ann...
Text Solution
|
- Which of the following pairs have approximately the same atomic radii?
Text Solution
|
- The correct order of radiii is/are:
Text Solution
|
- The first ionisation energy of first atom is greater than that of seco...
Text Solution
|
- Ionization energy of an element is:
Text Solution
|
- Consider the following ionization stesps: M(g) ot M^(+)(g)+e^(-)," ...
Text Solution
|
- Select the correct order of periodic properties of sepecies:
Text Solution
|