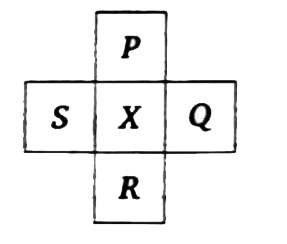
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCHTHE COLUMN|11 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|36 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise Level 3 (Passage Type)|89 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|4 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-PERIODIC PROPERTIES-ONE OR MORE ANSWERS IN/ARE CORRECT
- The properties which are common to both groups 1 and 17 elements in th...
Text Solution
|
- There are three elements A, B and C. their atomic number are Z(1), Z(2...
Text Solution
|
- Consider the following representation based on long form of periodic t...
Text Solution
|
- Which of the following match is/are correct regarding B, Al, C and S e...
Text Solution
|
- Consider the value of all four quantum number for last electron and sp...
Text Solution
|
- An element 'X' present in its ground state, the value of principal ann...
Text Solution
|
- Which of the following pairs have approximately the same atomic radii?
Text Solution
|
- The correct order of radiii is/are:
Text Solution
|
- The first ionisation energy of first atom is greater than that of seco...
Text Solution
|
- Ionization energy of an element is:
Text Solution
|
- Consider the following ionization stesps: M(g) ot M^(+)(g)+e^(-)," ...
Text Solution
|
- Select the correct order of periodic properties of sepecies:
Text Solution
|
- Select the incorrect statement(s)/order (s):
Text Solution
|
- Consider the following values of I.E.(eV) for elements W and X: {:("...
Text Solution
|
- The sum of IE(1) and IE(2), IE(3) and IE(4) for element P and Q are g...
Text Solution
|
- Consider the successive ionisation energy for an element 'A' IE(1),I...
Text Solution
|
- According to Slater's rule, correct order of Z(eff) on valence shell e...
Text Solution
|
- Which of the following order is/are correct?
Text Solution
|
- Correct order of electron affinity is/are:
Text Solution
|
- Which of the following statement(s) is/are correct?
Text Solution
|