Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise Level 2|102 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|11 VideosPERIODIC PROPERTIES
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|36 Videosp-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|35 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|4 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-PERIODIC PROPERTIES-SUBJECTIVE PROBLEMS
- If heat of solution for AB(s) is -0.95xx10^(x) kcal/mol and lattice en...
Text Solution
|
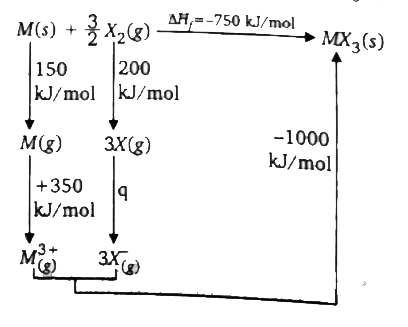
- Consider the following Borh-Habber's cycle for formation of MX(3)(s). ...
Text Solution
|
- Calculate value of -U/100, for AB(s), from following data of Born-Habe...
Text Solution
|
- Consider the following orders: (i) HF gt HCl gt HBr gt HI: Lewis bas...
Text Solution
|
- Find out total number of representative elements in the given element:...
Text Solution
|
- An element 'X' has its electronic configuration of 'K' shell is (n-5)s...
Text Solution
|
- if value of spin quantum number(s)=-1//2,0,+1//2 then calculate number...
Text Solution
|
- How many pairs are, in which first species has lower ionisation enegy ...
Text Solution
|
- Total number of element(s) which have only single oxidation state (oth...
Text Solution
|
- The number of electrons for Zn^(2+) cation that have the value of azim...
Text Solution
|
- Calculate the electronegativity of silicon atom using Allred-rochow's ...
Text Solution
|
- If heat of solution for AB(s) is -0.95xx10^(x) kcal/mol and lattice en...
Text Solution
|
- Consider the following Borh-Habber's cycle for formation of MX(3)(s). ...
Text Solution
|
- Calculate value of -U/100, for AB(s), from following data of Born-Habe...
Text Solution
|
- Consider the following orders: (i) HF gt HCl gt HBr gt HI: Lewis bas...
Text Solution
|
- Find out total number of representative elements in the given element:...
Text Solution
|
- An element 'X' has its electronic configuration of 'K' shell is (n-5)s...
Text Solution
|
- if value of spin quantum number(s)=-1//2,0,+1//2 then calculate number...
Text Solution
|
- How many pairs are, in which first species has lower ionisation enegy ...
Text Solution
|
- Total number of element(s) which have only single oxidation state (oth...
Text Solution
|