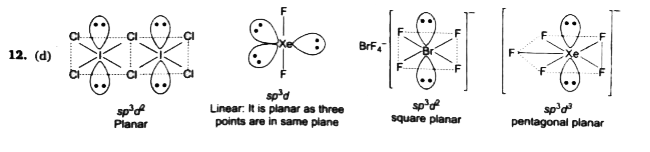A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise Level 3|77 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|78 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|64 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|54 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-Level 2
- Out of CHCl(3), CH(4) and SF(4) the molecules do not having regular ge...
Text Solution
|
- When iodine is dissolved in aqueous potassium iodide, the shape of the...
Text Solution
|
- Which of the following set of species have planar structure?
Text Solution
|
- Which of the following compounds have the same no. of lone pairs with ...
Text Solution
|
- Given the correct order of initials T or F for following statements. U...
Text Solution
|
- The correct sequence of polarity of the following molecule (1)Benzen...
Text Solution
|
- Which among the followiing molecules is not perfect flat?
Text Solution
|
- Which of the following structure(s) is /are non-planar?
Text Solution
|
- Nodal planes of pi-bonds in CH(2)=C=C=CH(2) are located in,
Text Solution
|
- Which of the following have X-O-X linkage ? (where X is central atom...
Text Solution
|
- Select the correct statements:
Text Solution
|
- Oxidation state of 'S' in peroxodisulphuric acid and sodium tetrathion...
Text Solution
|
- Structure of Na(2)[B(4)O(5)(OH)(4)]*8H(2)O contains
Text Solution
|
- Which of the following molecular species is not linear?
Text Solution
|
- Incorrect match is :
Text Solution
|
- Consider the following reactions: MX(4)+X'(2) to MX(4)X(2)' If ato...
Text Solution
|
- Select the correct statements:
Text Solution
|
- In which of the following species, d-obitals having xz an yz two nodal...
Text Solution
|
- The correct order of increasing s-character (in percentage) in the hyb...
Text Solution
|
- The shapes of MnO(4)^(-) ion and the hybridization of Mn in MnO(4)^(-)...
Text Solution
|