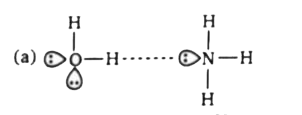A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise Level 3|77 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|78 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|64 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|54 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-Level 2
- The correct order of boiling point is:
Text Solution
|
- Cis butene dioic acid overset(K(a(1))(-H^(+)))overset(to)(larr)X(1)^(-...
Text Solution
|
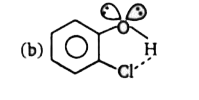
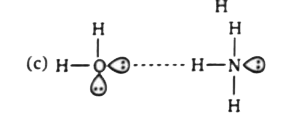
- Which of the following is not a best representation of the H-bond?
Text Solution
|
- The H-bonds in solid HF can be best represented as :
Text Solution
|
- Major product of the following reactions is
Text Solution
|
- Which of the following interaction lies in the range of 8-42kJ/mol?
Text Solution
|
- Assertion (A) : The presence of CO(2) in the air accelerates corrosio...
Text Solution
|
- The correct solubility order is/are (I) CaCO(3)gtSrCO(3)gtBaCO(3) ...
Text Solution
|
- On heating to 400-500^(@)C, relatively unstable hydrides and carbonate...
Text Solution
|
- Both N(SiH(3))(3) and NH(SiH(3))(2) compounds have trigonal planar ske...
Text Solution
|
- The incorrect statement regarding O(SiH(3))(2) and OCl(2) molecule is/...
Text Solution
|
- Among following molecule N-Si bond length is shortest:
Text Solution
|
- Which of the following molecule has weakest (ppi-dpi) back bonding?
Text Solution
|
- The geometry with respect to the central atom of the following molecul...
Text Solution
|
- Incorrect statement regarding BF(3)NH(3) molecule is :
Text Solution
|
- In which of the following molecules mu(oberved) is found to be greater...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Which of the following is wheat fruit?
Text Solution
|
- Which of the following is wheat fruit?
Text Solution
|
- The bond having the minimum bond energy is :
Text Solution
|