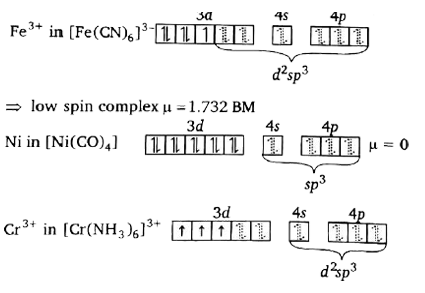
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
VK JAISWAL ENGLISH|Exercise LEVEL 3 (PASSAGE TYPE)|98 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWERS IS/ARE CORRECT|71 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|54 Videosd-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|32 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CO-ORDINATION COMPOUNDS-LEVEL 2
- Which of the followimg complex compound(s) is/are paramagnetic and low...
Text Solution
|
- Which of the following ligand does not as pi-acid ligand?
Text Solution
|
- If EAN of central metal cation M^(2+) in an non-chelating complex is 3...
Text Solution
|
- [Mn(CO)(4)NO] is diamagnetic because:
Text Solution
|
- Choose the correct option regarding the following complex compound whi...
Text Solution
|
- If CO ligands are substituted by NO in respective neutral carbonyl com...
Text Solution
|
- Which of the following species can act as reducing agent ?
Text Solution
|
- If Delta(0) lt P, the correct electronic configuration for d^(4) syste...
Text Solution
|
- Which of the following statement is not correct?
Text Solution
|
- Give the correct of initials T or F for following statements. Use T if...
Text Solution
|
- match List-I with list-II and select the correct answer using the code...
Text Solution
|
- The value of 'spin only' magnetic moment for one of the following conf...
Text Solution
|
- The correct order of magnetic moments (spin values in B.M.) among is:
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Set of d-orbitals which is used by central metal during formation of M...
Text Solution
|
- FeSO(4) is a very good absorber for NO, the new compound formed by thi...
Text Solution
|
- A [M(H(2)O)(6)]^(2+) complex typically absorbs at around 600 n. it is ...
Text Solution
|
- Given that the energy of the photons of different colours decreases in...
Text Solution
|
- The CFSE for octahedral [CoCl(6)]^(4-) is 18,000 cm^(-1) . The CFSE fo...
Text Solution
|
- MnO(4)^(-) is of intense pink colour, though Mn is in(+7) oxidation st...
Text Solution
|
- In which of the following complex ion the value of magnetic moment (sp...
Text Solution
|