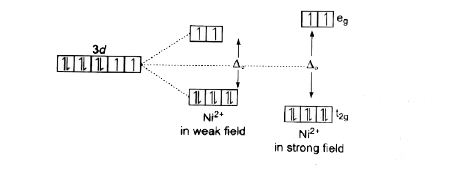
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CO-ORDINATION COMPOUNDS-LEVEL 2
- What is electronic arrangement of metal atom/ion in octahedral complex...
Text Solution
|
- Which of the following statement is not correct?
Text Solution
|
- Give the correct of initials T or F for following statements. Use T if...
Text Solution
|
- match List-I with list-II and select the correct answer using the code...
Text Solution
|
- The value of the spin only magnetic moment for one of the following co...
Text Solution
|
- The correct order of magnetic moments (spin values in BM) among is:
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Set of d-orbitals which is used by central metal during formation of M...
Text Solution
|
- FeSO(4) is a very good absorber for NO, the new compound formed by thi...
Text Solution
|
- A [M(H(2)O)(6)]^(2+) complex typically absorbs at around 600 nm. it is...
Text Solution
|
- An ion M^(2+), forms the complexes [M(H(2)O)(6)]^(2+),[M(en)(3)]^(2+) ...
Text Solution
|
- The SCSE for [(CoCl)(6)]^(4-) complex is 18000 cm^(-1). The Delta for ...
Text Solution
|
- MnO(4)^(-) is of intense pink colour, though Mn is in (+7) oxidation s...
Text Solution
|
- In which of the following complex ion the value of magnetic moment (sp...
Text Solution
|
- Which of the following order of CFSE is incorrect?
Text Solution
|
- For which of the following d^(n) configuration of octahedral complex(e...
Text Solution
|
- Consider the following: [Co(CO(3))(NH(3))(5)]ClO(4) mark the correct ...
Text Solution
|
- The pi acid ligand which uses it d-orbital during synergic bonding in ...
Text Solution
|
- The IR stretching frequencies of free CO, and CO in [V(CO)(6)]^(-),[C...
Text Solution
|
- The pi acid ligand which uses it d-orbital during synergic bonding in ...
Text Solution
|