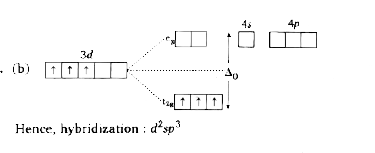
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CO-ORDINATION COMPOUNDS-LEVEL 2
- Match List-I and Li-st II and select the correct answer using the code...
Text Solution
|
- Which of the following is correct matched?
Text Solution
|
- The hybridization of the complex [CrCl(2)(NO(2))(2)(NH(3))(2)]^(-) is:
Text Solution
|
- Which of the following statement is not true for the reaction given be...
Text Solution
|
- Which of the following match is incorrect?
Text Solution
|
- Select the correct code of TRUE and FALSE for given statements: (a) ...
Text Solution
|
- The total possible coordination isomers for the following compounds re...
Text Solution
|
- Select the incorrect match:
Text Solution
|
- Select incorrect statement about complex [Cr(NO(2))(NH(3))(5)][Zn(SCN)...
Text Solution
|
- The magnetic property, dipole moment, plane of symmetry, colour and ab...
Text Solution
|
- Which of the following isomerism is not possible for complexes having ...
Text Solution
|
- Unmatched characteristic of complex [PdCl(2)(H(2)O)(2)(NH(3))(2)]^(2+)...
Text Solution
|
- Which of the following has largest number of isomers?
Text Solution
|
- Which one of the following complexes does not exhibit chirality?
Text Solution
|
- Consider the following isomerism: (i) Ionization (ii) Hydrate (i...
Text Solution
|
- Which complex is likely to show optical activity?
Text Solution
|
- Which of the following statement is true?
Text Solution
|
- The following complexes are given: (1) trans-[Co(NH(3))(4)Cl(2)]^(+)...
Text Solution
|
- Which of the following can show geometrical isomerism?
Text Solution
|
- Which of the following complex compound exhibits cis-trans isomerism?
Text Solution
|