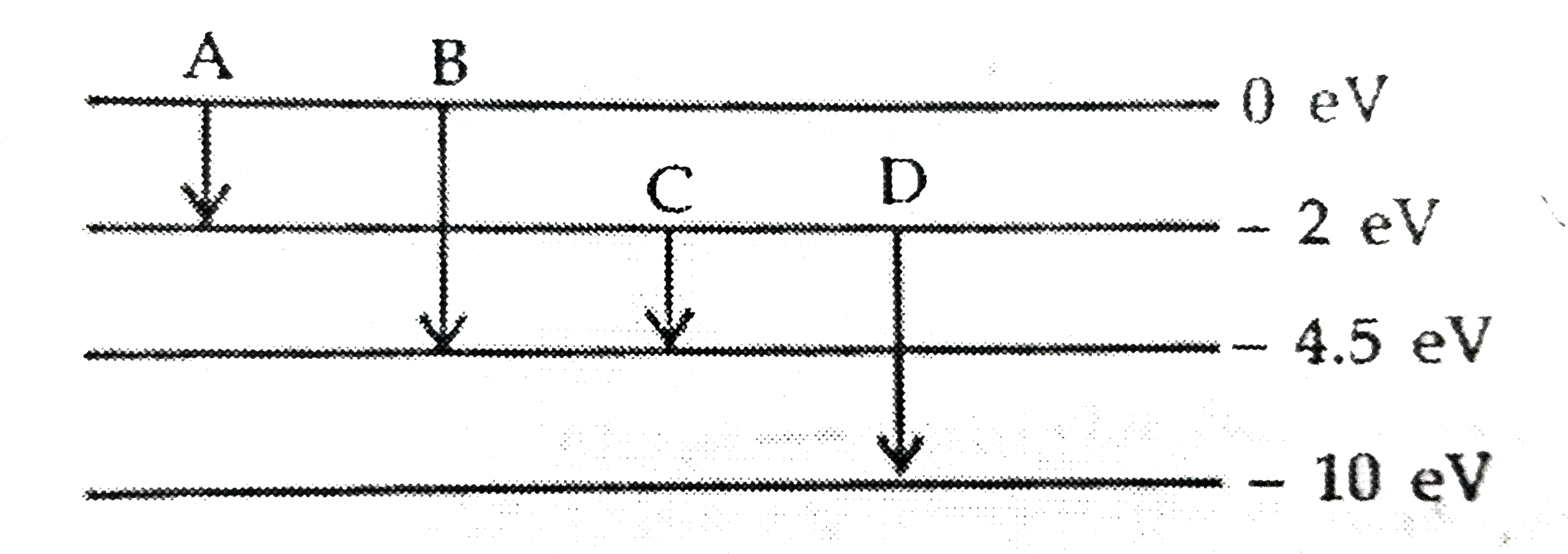
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Exercise 4.2|12 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Subject|17 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Solved Examples|23 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-Exercise 4.1
- State the Following statement as statements as TRUE and FALSE. a. T...
Text Solution
|
- Transition between three energy energy levels in a particular atom gi...
Text Solution
|
- A hydrogen atom in a having a binding of 0.85eVmakes transition to a s...
Text Solution
|
- Calculate (a) the wavelength and (b) the frequencey of the Hbeta line...
Text Solution
|
- Find the longest and shortest wavelengths in the Lyman series for hy...
Text Solution
|
- Using the known values for haydrogen atom, calculate . (a) radius of t...
Text Solution
|
- A small particle of mass m moves in such a way that the potential en...
Text Solution
|
- An imaginary particle has a charge equal to that of an electron and m...
Text Solution
|
- Hydrogen gas in the atomic state is excited to an energy level such th...
Text Solution
|
- Taking into account the motion of the nucleus of a hydrogen atom , fin...
Text Solution
|
- An electron having energy 20 e V collides with a hydrogen atom in the ...
Text Solution
|
- According to classical physics, an electron in periodic motion with em...
Text Solution
|
- (a) The enegy levels of an atom are as shown below. Which of them will...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. (i) What is...
Text Solution
|
- Suppose that means were available for stripping 28 electrons from 29 C...
Text Solution
|