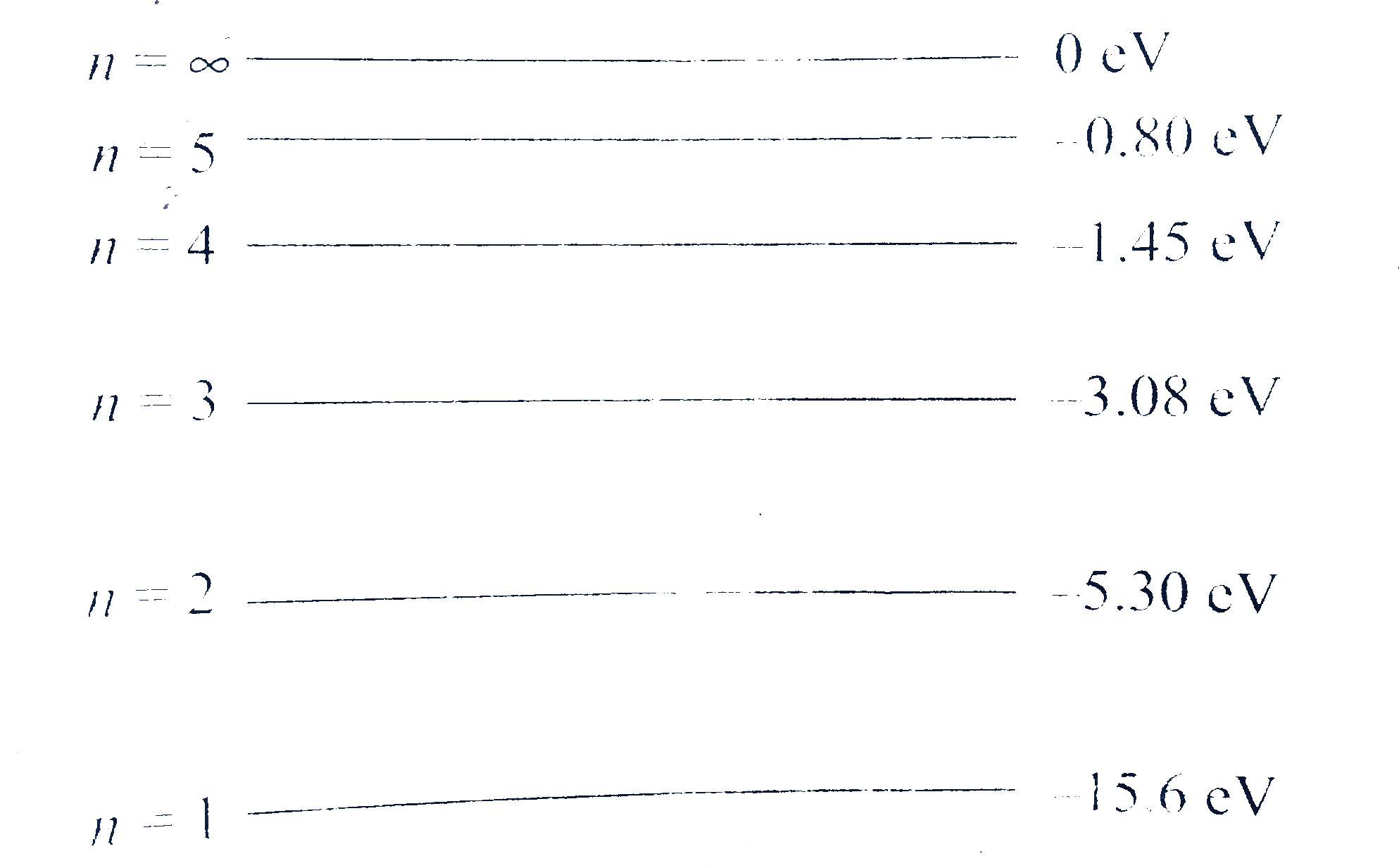
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Integer|4 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Fill In The Blanks|8 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|13 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-Linked Comprehension
- The electrons in a H- atom kept at rest , jumps from the mth shell to ...
Text Solution
|
- The electrons in a H- atom kept at rest , jumps from the mth shell to ...
Text Solution
|
- The energy levels of a hypothetical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The electron in a hydrogen atom at rest makes a transition from n = 2 ...
Text Solution
|
- The electron in a hydrogen atom at rest makes a transition from n = 2 ...
Text Solution
|
- For a certain hypothetical one electron atom, the wavelength (in Å) fo...
Text Solution
|
- For a certain hypothetical one electron atom, the wavelength (in Å) fo...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A sample of hydrogen gas in its ground state is irradiated with photon...
Text Solution
|
- A neutron of kinetic 65 eV collides inelastically with a singly ionize...
Text Solution
|
- A neutron of kinetic 6.5 eV collides inelastically with a singly ioniz...
Text Solution
|
- Soppose potential energy between electronand proton at seperation r is...
Text Solution
|
- Soppose potential energy between electronand proton at seperation r is...
Text Solution
|