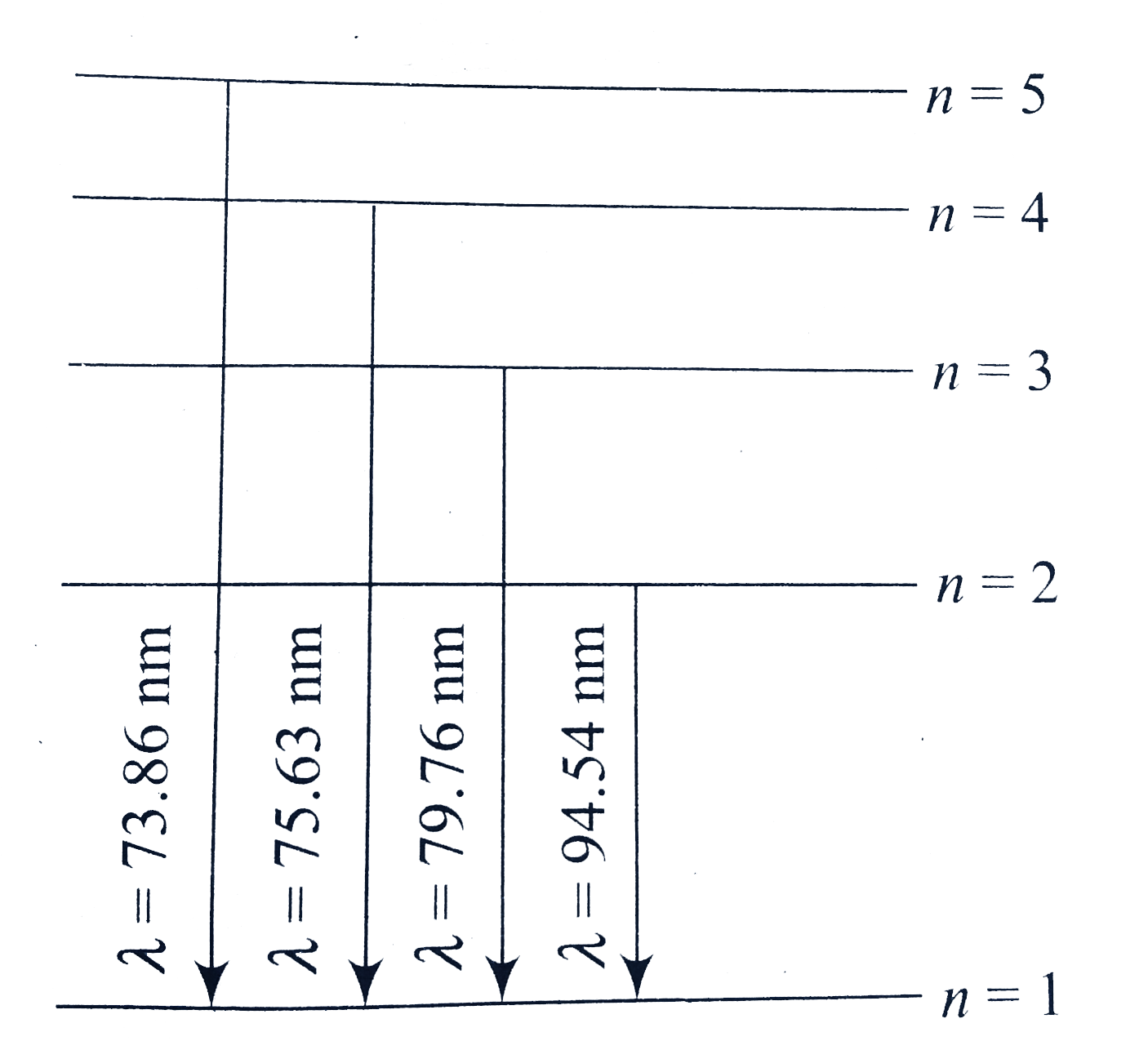
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Integer|4 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Fill In The Blanks|8 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|13 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-Linked Comprehension
- When high energetic electron beam , (i.e., cathode rays) strike the he...
Text Solution
|
- Light form a dicharge tube containing hydrogen atoms falls on the surf...
Text Solution
|
- Light form a dicharge tube containing hydrogen atoms falls on the surf...
Text Solution
|
- Light form a dicharge tube containing hydrogen atoms falls on the surf...
Text Solution
|
- The electron in a Li^(+ +) ion is the nth shell , n being very large. ...
Text Solution
|
- The electron in a Li^(+ +) ion is the nth shell , n being very large. ...
Text Solution
|
- The electron in a Li^(+ +) ion is the nth shell , n being very large. ...
Text Solution
|
- Two hydrogen-like atoms A and B are of different masses and each atom ...
Text Solution
|
- Two hydrogen-like atoms A and B are of different masses and each atom ...
Text Solution
|
- 1.8 g of hydrogen is excite by irradiation. The study of spectra indic...
Text Solution
|
- 1.8 g of hydrogen is excite by irradiation. The study of spectra indic...
Text Solution
|
- In a set of experiment on a hydrogen on a hypotherical one-electron at...
Text Solution
|
- In a set of experiment on a hydrogen on a hypotherical one-electron at...
Text Solution
|
- In a set of experiment on a hydrogen on a hypotherical one-electron at...
Text Solution
|
- The recoil speed of a hydrogen atom after it emits a photon is going...
Text Solution
|
- Kalpha wavelength emitted by an atom of atomic number Z=11 is lambda. ...
Text Solution
|
- In a hydrogen atom, the electron is in nth excited it comes down to fi...
Text Solution
|
- The shortest wavelength of the Brackett series of hydrogen like atom ...
Text Solution
|
- Heat at the rate of 200 W is produced in an X-ray tube operating at 20...
Text Solution
|
- An electron in an H-atom kept at rest , jumps from the mth shell to th...
Text Solution
|