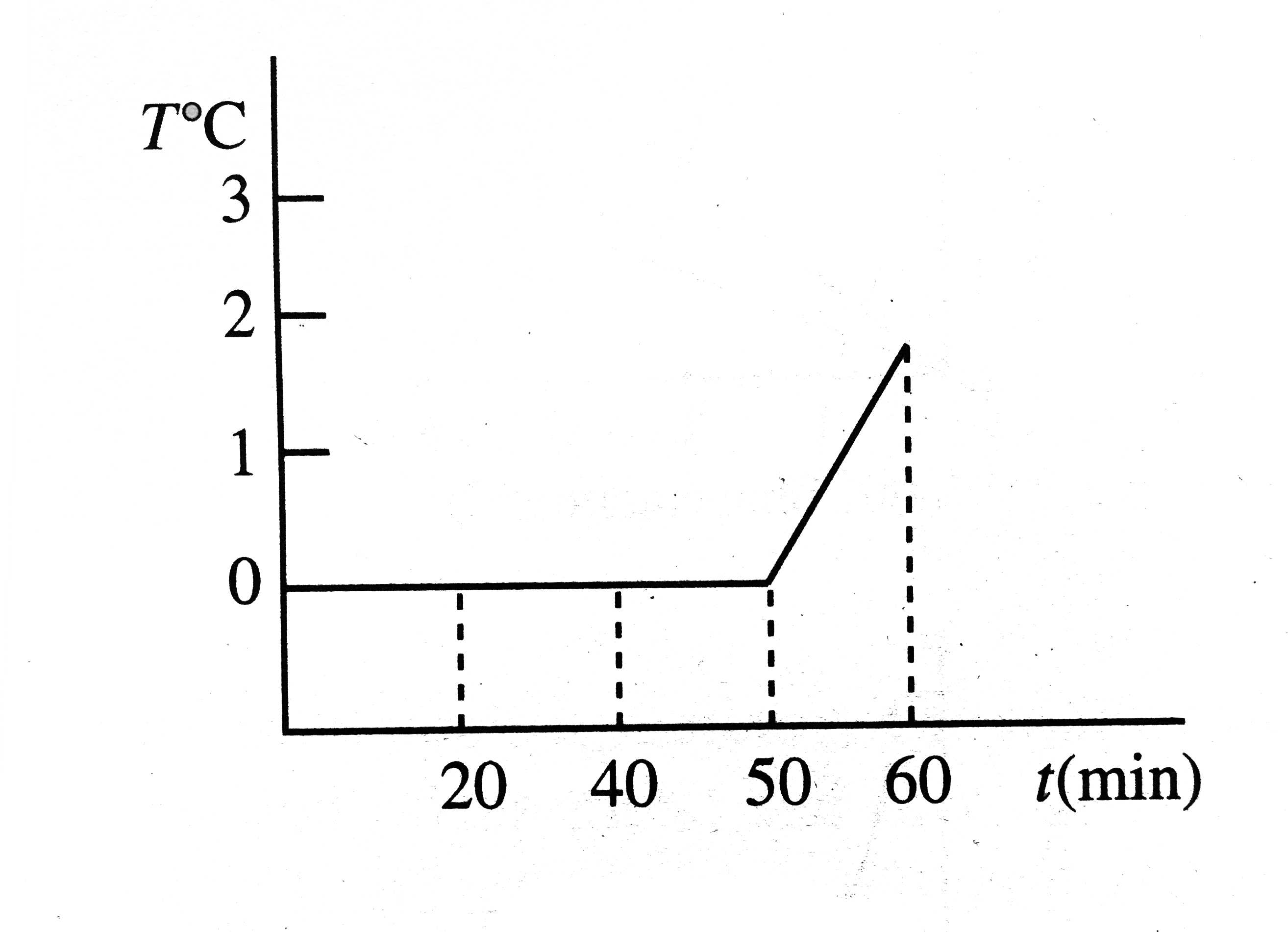A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CALORIMETRY
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|25 VideosCALORIMETRY
CENGAGE PHYSICS ENGLISH|Exercise Comprehension|30 VideosCALORIMETRY
CENGAGE PHYSICS ENGLISH|Exercise Subjective|25 VideosBASIC MATHEMATICS
CENGAGE PHYSICS ENGLISH|Exercise Exercise 2.6|20 VideosCENTRE OF MASS
CENGAGE PHYSICS ENGLISH|Exercise INTEGER_TYPE|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-CALORIMETRY-Single Correct
- A and B are tqo isolated spheres kept in close proximity so that they ...
Text Solution
|
- A thermally isulated piece of metal is heated under atmospheric pressu...
Text Solution
|
- A cooking vessel on a slow burner contains 5 kg of water and an unknow...
Text Solution
|
- A glass cylinder contains m0=100g of mercury at a temperature of t0=0^...
Text Solution
|
- Power radiated by a black body is P0 and the wavelength corresponding ...
Text Solution
|
- Three rods AB, BC and BD of same length l and cross section A are arra...
Text Solution
|
- The length of s steel rod exceeds that of a brass rod by 5 cm. If the ...
Text Solution
|
- A container of capacity 700 mL is filled with two immiscible liquids o...
Text Solution
|
- The coefficient of apparent expansion of a liquid when determined usin...
Text Solution
|
- A glass vessel is filled up to 3/5th of its volume by mercury. If the ...
Text Solution
|
- A thin circular metal disc of radius 500.0 mm is set rotating about a ...
Text Solution
|
- A platinum sphere floats in mercury. Find the percentage change in the...
Text Solution
|
- The variation of length of two metal rods A and B with change in tempe...
Text Solution
|
- The graph of elongation of rod of a substance A with temperature rise ...
Text Solution
|
- Figure. Shows the graphs of elongation versus temperature for two diff...
Text Solution
|
- Water at 0^@C contained in a closed vessel, is abruptly opened in an e...
Text Solution
|
- A system receives heat continuously at the rate of 10 W. The temperatu...
Text Solution
|
- In a motor the electrical power input is 500 W and the mechanical powe...
Text Solution
|
- The molar heat capacity of a certain substance varies with temperature...
Text Solution
|
- The corfficient of linear expansion for a certain metal varies with te...
Text Solution
|