Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ARCHIVES 1 VOLUME 6-Integer
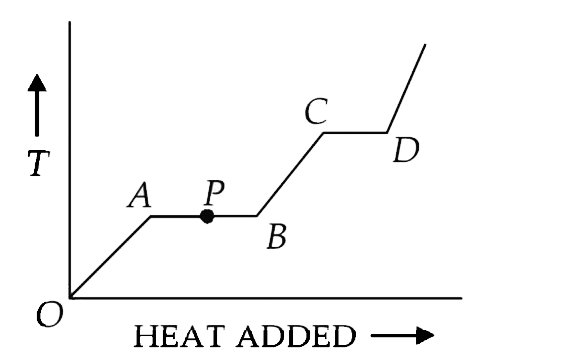
- The variation of temperature of a material as heat is given to it at a...
Text Solution
|
- A metal rod AB of length 10x has its one end A in ice at 0^@C, and the...
Text Solution
|
- Two spherical black bodies of radii r(1) and r(2) and with surface tem...
Text Solution
|
- A diatomic ideal gas is compressed adiabatically to 1/32 of its initia...
Text Solution
|
- Steel wire of length 'L' at 40^@C is suspended from the ceiling and th...
Text Solution
|