A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise dpp 4.2|15 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise ddp.4.3|15 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Linked comprehension type|6 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-dpp-4.1
- The figure shows a graph between 1n |(A(n))/(A(1))| and 1n|n|, where ...
Text Solution
|
- The force acting on the electron in a hydrogen atom depends on the pri...
Text Solution
|
- Ionization potential of hydrogen atom is 13.6eV . Hydrogen atoms in th...
Text Solution
|
- Find the ratio of ionization energy of bohr 's hydrogen atom doubly li...
Text Solution
|
- What is the angular momentum of an electron in Bohr's hydrogen atom wh...
Text Solution
|
- The ratio between total acceleration of the electron in singly ionized...
Text Solution
|
- A hydrogen atom in ground state absorbs 10.2eV of energy .The orbital ...
Text Solution
|
- Thr ratio of the speed of the electron in the ground state of hydrogen...
Text Solution
|
- Minimum excitation potential of Bohr's first orbit hydrogen atom is
Text Solution
|
- Which of the following statement is true regarding Bohr's model of hyd...
Text Solution
|
- The de- broglie wavelength of an electron in the first bohr orbit is
Text Solution
|
- In a hypothetical Bohr hydrogen, the mass of the electron is doubled. ...
Text Solution
|
- Which of the following transitions gives photon of maximum energy?
Text Solution
|
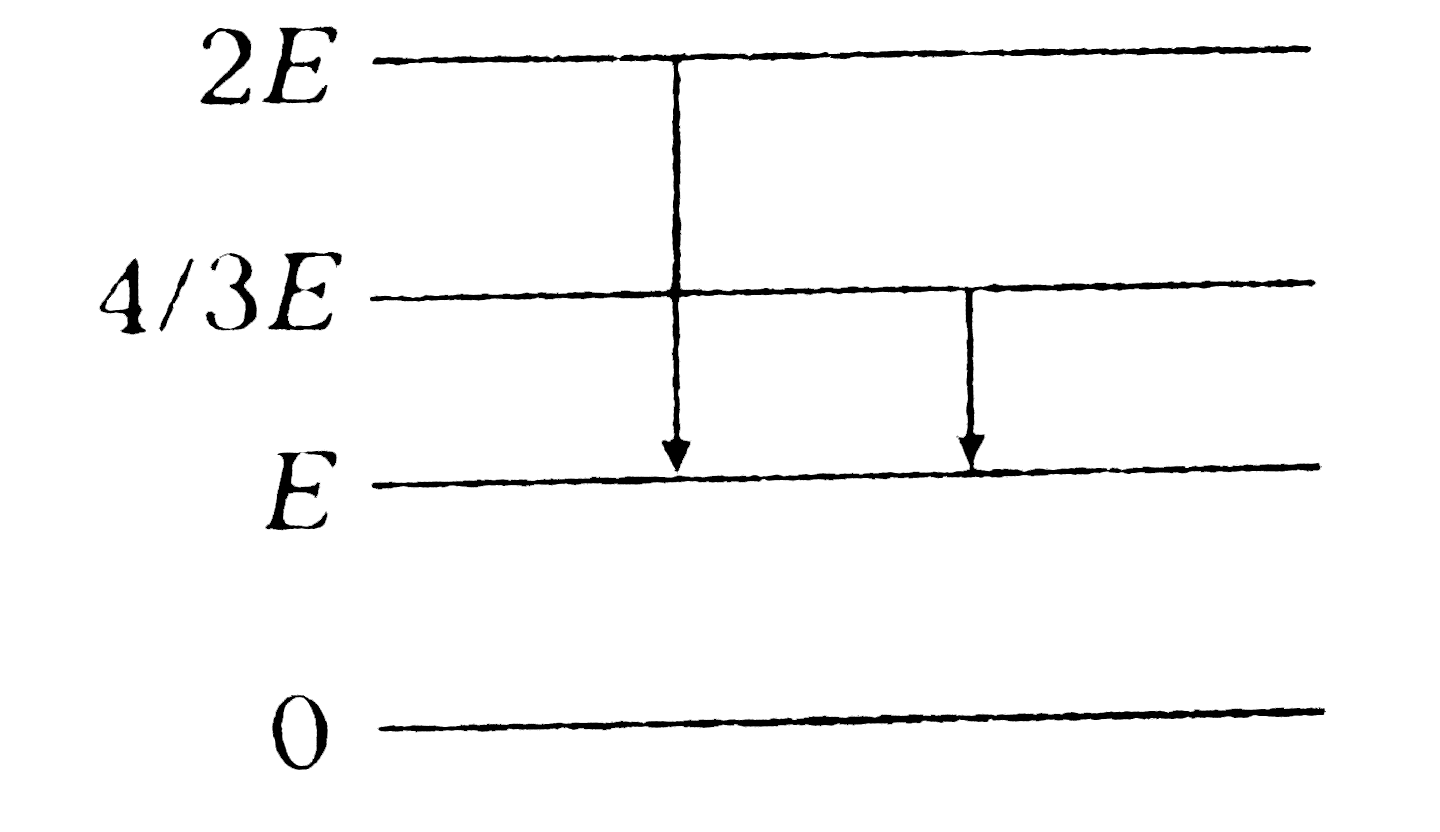
- The energy levels of a certain atom are represented in adjoining figur...
Text Solution
|
- A doubly ionized lithium atom is hydrogen like with atomic number 3. F...
Text Solution
|