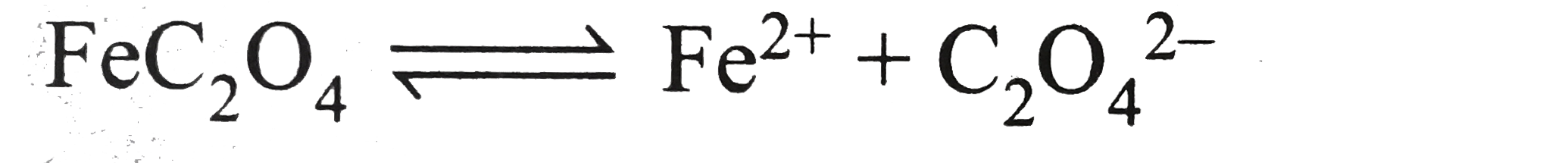A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion Reasoning|15 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer|16 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|30 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Ture False)|25 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STOICHIOMETRY-Exercises Single Correct
- 0.5 g of an organic substance containing phosphorus was heated with co...
Text Solution
|
- A bolloon blown up has a volume of 300 mL at 27% C. The balloon is dis...
Text Solution
|
- The number of moles of KMnO4 that are needed to react completely with ...
Text Solution
|
- In a reaction, 4 mole of electrons are transferred to 1 mole of HNO(3)...
Text Solution
|
- If equal volumes of 1 M KMnO(4) and 1 M K(2)Cr(2)O(7) solution are use...
Text Solution
|
- SO(2)Cl(2) (sulphuryl chloride ) reacts with water to given a mixture ...
Text Solution
|
- 100 mL of 0.01 M KMnO(4) oxidised 100 mL H(2)O(2) in acidic medium. ...
Text Solution
|
- 10mL of H2O2 solution (volume strength = x) requires 10mL of N//0.56 M...
Text Solution
|
- How many moles of HgI4^(2-) will be formed when 1 " mol of "Hg^(2+) an...
Text Solution
|
- When 1xx10^(-3) " mol of "the chloride of an elements Y was completely...
Text Solution
|
- In the mixture of (NaHCO3 + Na2CO3), volume of HCI required is x mL wi...
Text Solution
|
- Equivalent mass of KMnO4 in acidic, strongly basic and neutral are in ...
Text Solution
|
- NH(3)+Ocl^(ɵ)toN(2)H(4)+Cl^(ɵ) On balancing the above equation in ba...
Text Solution
|
- 50 mL of 0.1 M solution of a salt reacted with 25 mL of 0.1 M solution...
Text Solution
|
- 20 " mL of " x M HCl neutralises completely 10 " mL of " 0.1 M NaHCO3 ...
Text Solution
|
- Atomic weight of barium is 137.34 The equivalent weight of barium is B...
Text Solution
|
- The normality and volume strength of a solution made by mixing 1.0 L e...
Text Solution
|
- 36 " mL of " 0.5 M Br(2) solution when made alkaline undergoes complet...
Text Solution
|
- RH(2) ( ion exchange resin) can replace Ca^(2+)d in hard water as. R...
Text Solution
|
- 100mL of H2O2 is oxidised by 100mL of 0.01M KMnO4 in acidic medium (Mn...
Text Solution
|