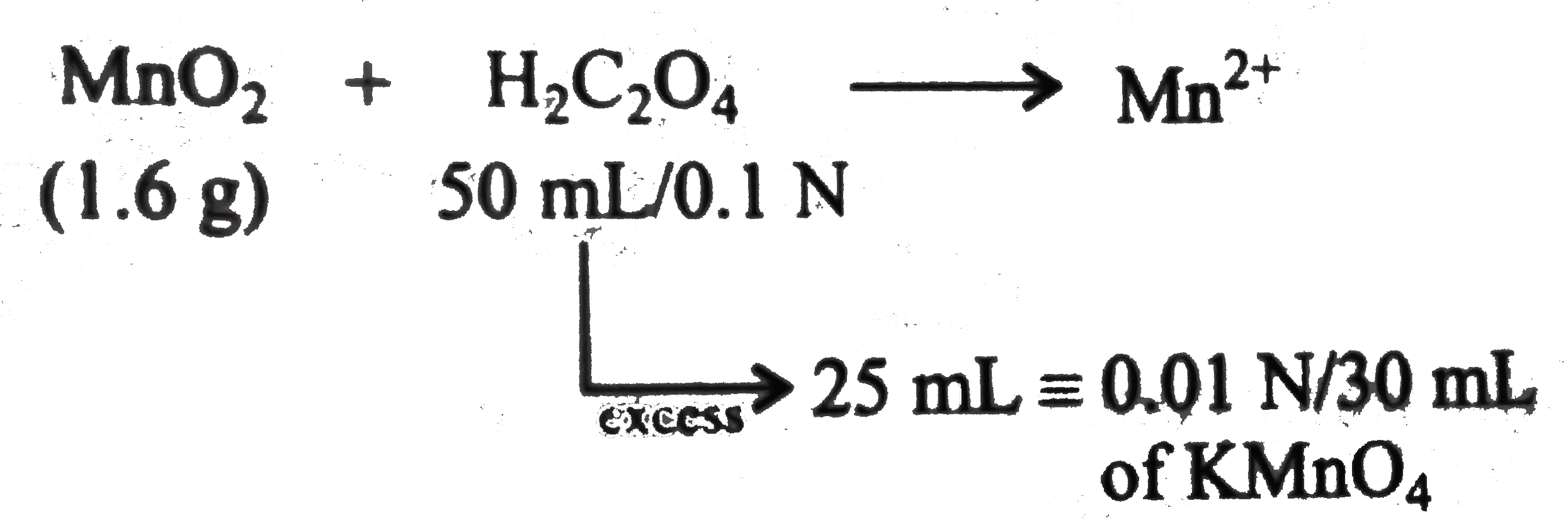A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion Reasoning|15 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer|16 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|30 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Ture False)|25 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STOICHIOMETRY-Exercises Single Correct
- What volume of 0.2 M KMnO(4) is required to react with 1.58 g of hypo ...
Text Solution
|
- Certain " mol of "HCn is oxidised completely by 25 " mL of " KMnO(4). ...
Text Solution
|
- 0.6 g of a sample of pyrolusite was boiled with 200 " mL of " (N)/(10)...
Text Solution
|
- In basic solution CrO4^(2-) oxidises S2O3^(2-) to form [Cr(OH)4]^(ɵ) a...
Text Solution
|
- KI reacts with H(2)SO(4) producing I(2) and H(2)S the volume of 0.2 M ...
Text Solution
|
- A 0.46 g sample of As(2)O(3) required 25.0 " mL of " KMnO(4) solution ...
Text Solution
|
- The number of moles of K(2)Cr(2)O(7) reduced by one mole of Sn^(2+) i...
Text Solution
|
- 10 " mL of " H(2)O(2) solution (volume strength =x) required 10 " mL o...
Text Solution
|
- Equivalent mass of H(3)PO(2) when it disproportionate into PH(3) and H...
Text Solution
|
- What volume of 0.1 M Ca(OH)(2) will be required neutralise 200 " mL of...
Text Solution
|
- What volume of 0.1 M Ca(OH)(2) will be required to neutralise 200 " mL...
Text Solution
|
- What volume of 0.2 M KOH will be requried to neutralise 100 " mL of " ...
Text Solution
|
- What volume of 0.1 M Ba(OH)(2) will be required to neutralise a mixtur...
Text Solution
|
- When 100 " mL of " 0.1 M Ba(OH)(2) is neutralised with a mixture of x ...
Text Solution
|
- If 10g of V(2)O(5) is dissolved in acid and is reduced to V^(2+) by zi...
Text Solution
|
- The volume of 0.5 M H(3)PO(4) that completely dissolved 3.1 g of coppe...
Text Solution
|
- 1 g of a sample of NaOH was dissolved in 50 " mL of " 0.33 M alkaline ...
Text Solution
|
- 0.4 g of polybasic acid HnA (all the hydrogens are acidic) requries 0....
Text Solution
|
- A mixutre solution of KOH and Na2CO3 requires 15 " mL of " (N)/(20) HC...
Text Solution
|
- 25.0 g of FeSO(4).7H(2)O was dissolved in water containing dilute H(2)...
Text Solution
|