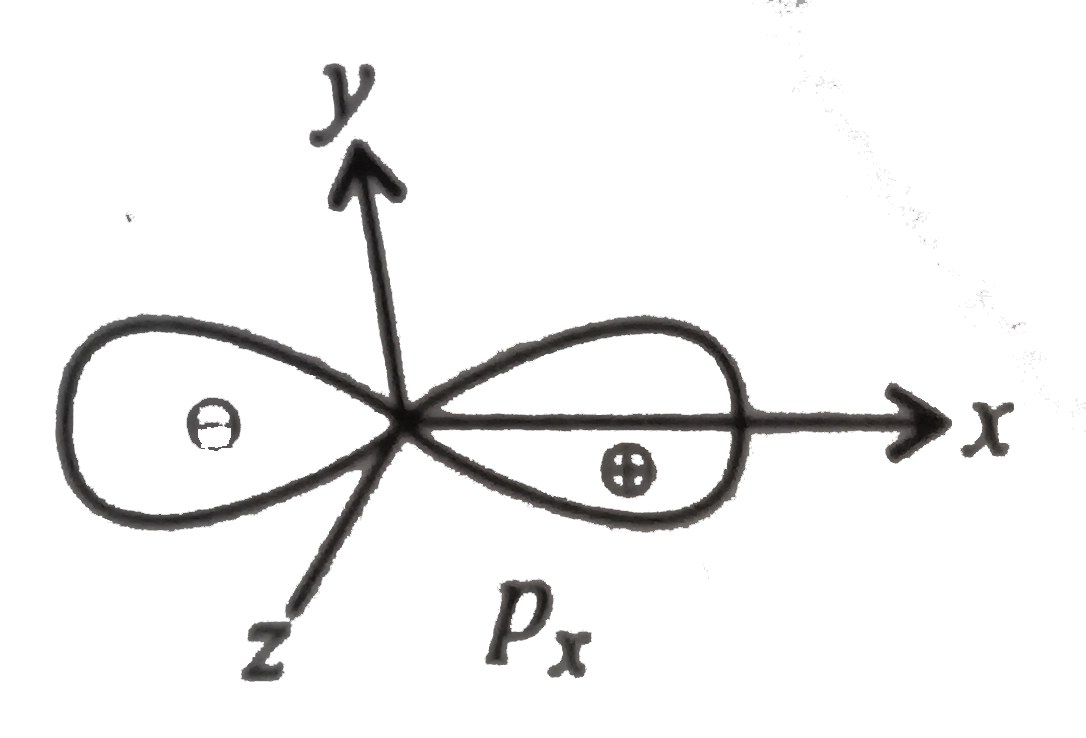
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Subjective)|52 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|50 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Concept Applicationexercise(4.3)
- The probability of fiating the electron in p(s) orbits is :
Text Solution
|
- How many quantum number are needed in designate an orbital ? Name the...
Text Solution
|
- The principal quantum number of n of an atomic orbitals is 5 what are ...
Text Solution
|
- (a) An atomic orbital has n=3. What are the possible values of l? (b...
Text Solution
|
- What is the lowest value of n that allows g orbital to exist?
Text Solution
|
- Given the notation for the sub-shell deotected by the following quant...
Text Solution
|
- How many electron on a fully filled f sub-shell have m(1) = 0 ?
Text Solution
|
- An electron is in one of the 3d orbitals. Give the possible values of ...
Text Solution
|
- If the largest value ofm(1) for an electron is + 3 in what type of su...
Text Solution
|
- Explain giving reasons, which of the following sets of quantum numbers...
Text Solution
|
- How many electron in atom may have the following quantum number ? A n ...
Text Solution
|
- How many orbitals are possible in a. 4th energy level b. 5f sub-she...
Text Solution
|
- What are the possible values of l and m(1) for an atomic orbital 4f?
Text Solution
|
- What is the shape of 1s and 2s orbital .Give two point of difference ...
Text Solution
|
- (a) How many sub-shells are associated with n = 4? (b) How many electr...
Text Solution
|
- How many spherical nodes are present in 4s orbital in a hydrogen ato...
Text Solution
|
- The principal quantum number representwsw
Text Solution
|
- The energy of an electron of 2p(1) orbital is
Text Solution
|
- The orbital angular momentum of an electron of an electron in 2s orbit...
Text Solution
|
- The number of angular nodal planes of zero electron density in the d(...
Text Solution
|