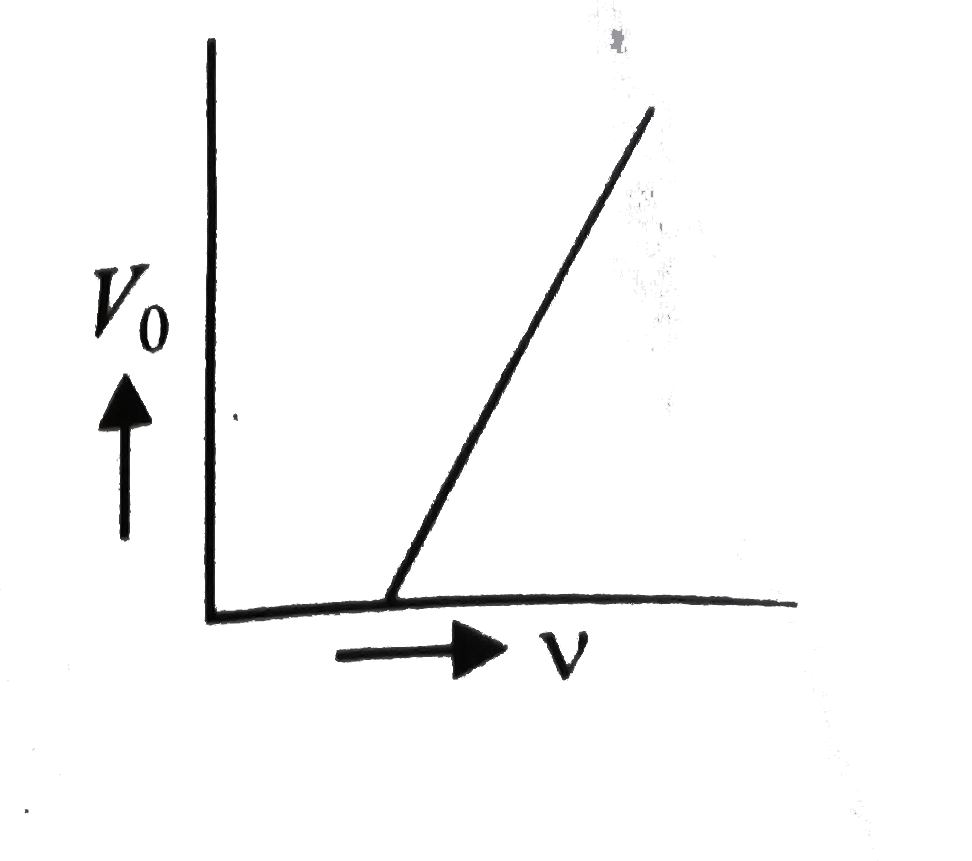
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|45 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|127 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Subjective)|52 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Exercises Linked Comprehension
- The sequence of filling electron in sub-shells of element with few ex...
Text Solution
|
- The sepence of filling electgron in sub-shells of element with few ex...
Text Solution
|
- The only element in the hydrogen atom resides under ordinary conditi...
Text Solution
|
- The only element in the hydrogen atom resides under ordinary conditi...
Text Solution
|
- The only element in the hydrogen atom resides under ordinary conditi...
Text Solution
|
- The only element in the hydrogen atom resides under ordinary conditi...
Text Solution
|
- The shape of orbitals are related to the ratio of principal quantum ...
Text Solution
|
- The shape of orbitals are related to the ratio of principal quantum ...
Text Solution
|
- The shape of orbitals are related to the ratio of principal quantum ...
Text Solution
|
- The shape of orbitals are related to the ratio of principal quantum ...
Text Solution
|
- The shape of orbitals are related to the ratio of principal quantum ...
Text Solution
|
- The emission of electrons from a metal surface exposed rto light r...
Text Solution
|
- The emission of electrons from a metal surface exposed rto light r...
Text Solution
|
- The emission of electrons from a metal surface exposed rto light r...
Text Solution
|
- The emission of electrons from a metal surface exposed rto light r...
Text Solution
|
- The emission of electrons from a metal surface exposed rto light r...
Text Solution
|
- It is tempting to think that all possible transition are permissible a...
Text Solution
|
- It is teming to think that all possible transituion are permissible an...
Text Solution
|
- The energy of state s(1) in units of the hydrogen atom ground state...
Text Solution
|
- The hydrogen -like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|